Powering a Connected World for Improved Human Health
We envision a future where data and intelligence accelerate innovation across the global healthcare ecosystem.
The World’s Broadest Federated Network of Global Real-World Data and Evidence for Life Sciences and Healthcare
TriNetX partners with leading healthcare organizations around the world to bring together the most comprehensive, privacy-compliant real-world data network. We apply advanced analytics and intelligence to help life sciences, researchers, and regulators accelerate breakthroughs in care.
Our Mission is Our Promise
At TriNetX, our mission is to operate the world’s broadest federated network of real-world data in partnership with healthcare providers and apply intelligence that accelerates innovation across the healthcare ecosystem.
Empowering Global Research: Leading the Way in Real-World Data for Comprehensive Drug Development
Beyond merely providing data, we offer end-to-end support throughout the drug development lifecycle. Our extensive research capabilities empower researchers to publish groundbreaking findings in prestigious medical journals while leveraging our platform technologies, integrated analytics, and downloadable datasets. From optimizing clinical trial designs to refining post-market safety measures, we offer comprehensive solutions that drive innovation and elevate healthcare standards worldwide. Join us in shaping a safer, healthier future, where every data point contributes to better patient outcomes and medical advancements.
Enhance Study Feasibility
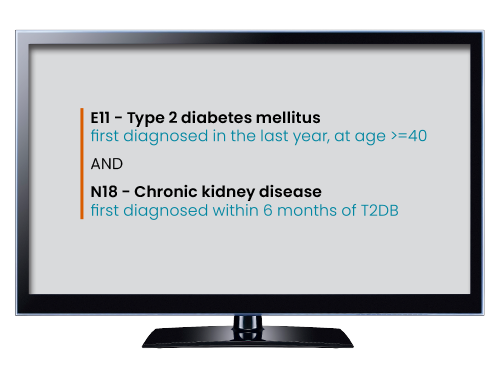
Evaluate criteria, comparators, and endpoints based on real-world data spanning domains from diagnoses to genomic variants. Leverage insights from real-world data (RWD) and real-world evidence (RWE) studies to enhance drug development and learn about real-world medicine and health. Test criteria against patients receiving care now.
Streamline Trial Site Collaboration
Utilize real-world data (RWD) and real-world evidence (RWE) to identify potential clinical trial sites and facilitate connections between sponsors and healthcare organizations serving patients seeking care.
Generate RWE & Unlock Insights
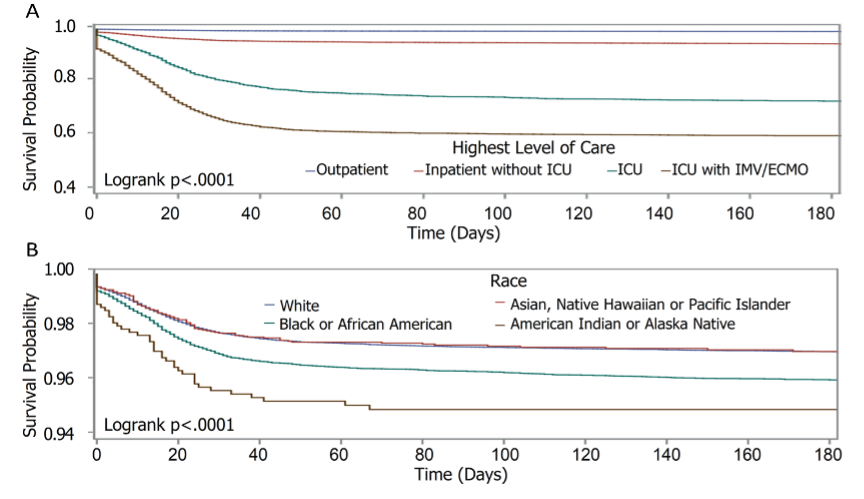
Answer questions of safety, efficacy, and value for all your stakeholders, from patients to regulators. Benefit from expert evidence strategists for optimized solutions leveraging real-world data to generate the insights for healthcare systems and clinical research.
Mitigate Drug Safety Signals
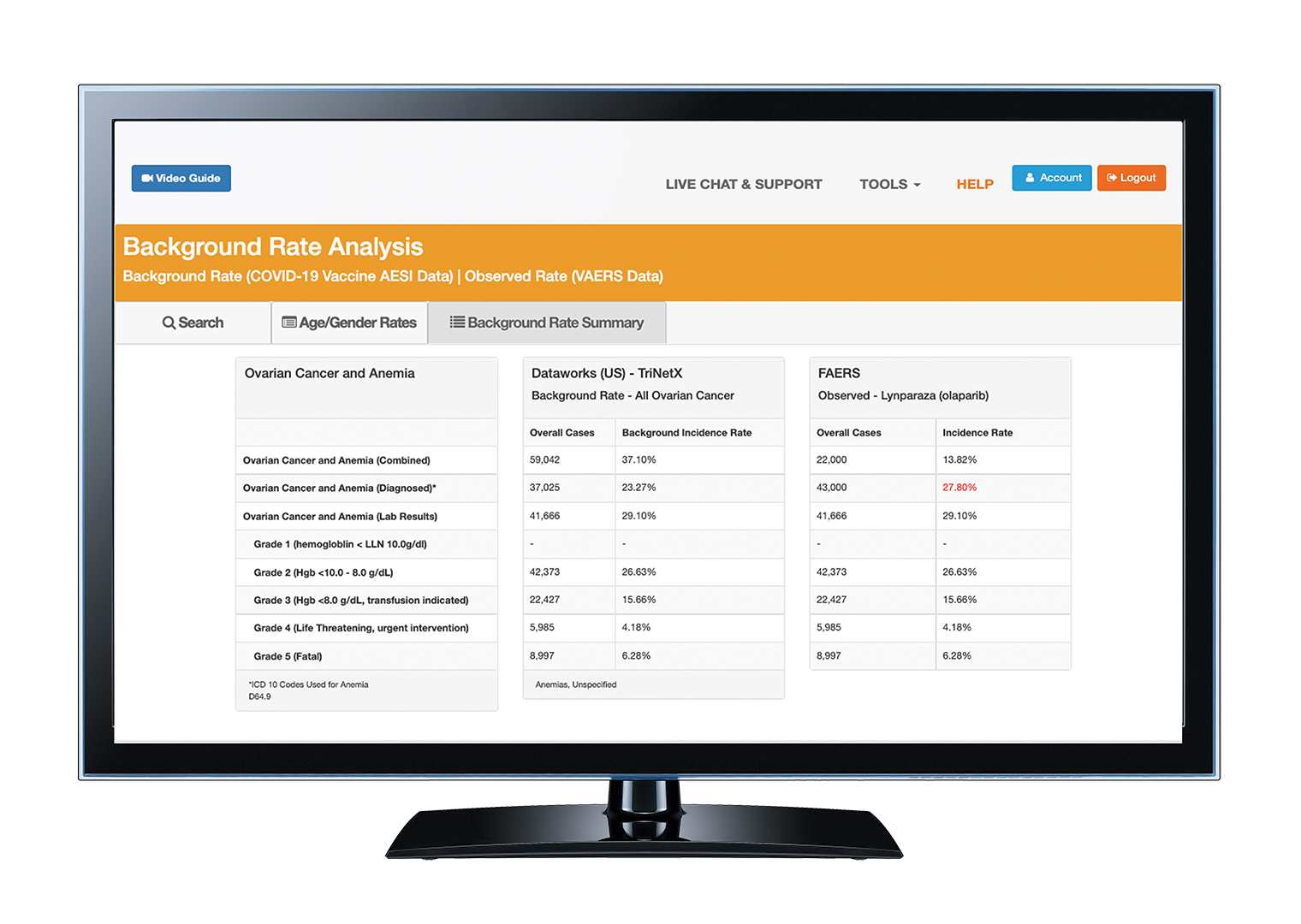
Discover the next generation of pharmacovigilance. Combine harmonized drug safety data from public sources with electronic health records (EHR) and claims to enhance signaling algorithms for drug safety.
From Insight to Impact—How We Help You Move Faster
Real-World Impact Across the Healthcare Ecosystem
Hear how TriNetX power users at Sanofi, the Medical University of South Carolina, and the University of Arkansas for Medical Sciences are conducting more, and more informed, research.
Real-world data and evidence that runs miles wide...
patient lives
healthcare organizations providing continuous, comprehensive, up-to-the-month data
countries in North and South America, EMEA, and Asia-Pacific
...and miles deep.
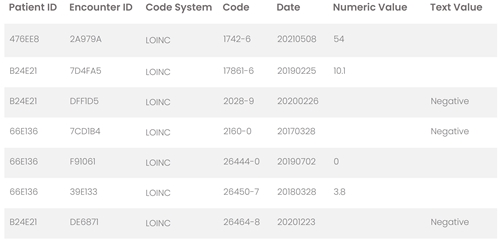
date- and patient-specific clinical observations available for download
lab results in the typical record of U.S. and EMEA patients
years of clinical history on more than 40 million patients around the globe
“Are you a software company? A data company? A consulting company?”
Yes, yes, and yes. To put it simply, we’re an answer company. TriNetX is committed to helping you unearth insights that will lead to better patient care and treatment options. How you get there depends on your challenge. Need a fast, reliable answer to an incidence or prevalence question? Turn to our platform-based analytics. Want to understand the patient journey, from changing creatinine levels to cost of care? Our linked EHR and claims dataset has what you need. Ready to collaborate with the sharpest minds in real-world data? Our consulting team can’t wait to join forces with you.

Keep updated with the latest TriNetX News and Insights
Finding the Unfindable: How One Healthcare Organization is Using TriNetX to Identify Underdiagnosed Heart Disease
We’re proud to announce Worcestershire Acute Hospitals NHS Trust as our Q1 2026 Network Spotlight winner! Led by Dr. David Wilson, Consultant Cardiologist...
TriNetX Unveils Conversational AI Interface and Enhanced API Capabilities
Success achieved in reducing trial costs and timelines drives 2026 platform enhancements designed to democratize clinical research analytics. CAMBRIDGE,...
The Year Real-World Data Became Essential: The Resources That Captivated Our Community in 2025
As we step into 2026, the biopharma industry finds itself at a different starting line than anyone predicted just a year ago. As Director of Content &...


 Victoria DiBiaso
Victoria DiBiaso Amy Jo Jenkins
Amy Jo Jenkins Signe Denmark, MS, CCRP
Signe Denmark, MS, CCRP