TriNetX Consulting Services
The right answers to your clinical questions, delivered by TriNetX expertise.
About TriNetX Consulting Services
Our team of analysts, epidemiologists, and evidence strategists represent decades of world-class training and experience. Partner with us to uncover solutions to your research challenges.
TriNetX Consulting Service Offerings
Non-US Oncology Analytics & Insights
Our European-based TriNetX Oncology team collaborates with renowned medical experts and pharmaceutical leaders to generate fit-for-purpose database specifications to match the desired study objectives scoping for oncological and rare-disease indications. By gathering complete, representative, and high-quality data, TherapyMonitor reflects the variety of cancer care across the continent.
Use Cases
-
- Health Economics Outcomes Research (HEOR)
- Market dynamics
- Treatment and therapy patterns
- Best achieved outcomes
Ready to see a sample TherapyMonitor Report?
TherapyMonitor projects are indication-specific, comprehensive analyses revealing current disease prevalence, treatment patterns, and indication-specific market dynamics based on longitudinal, patient-level data.
Design, Find, Select
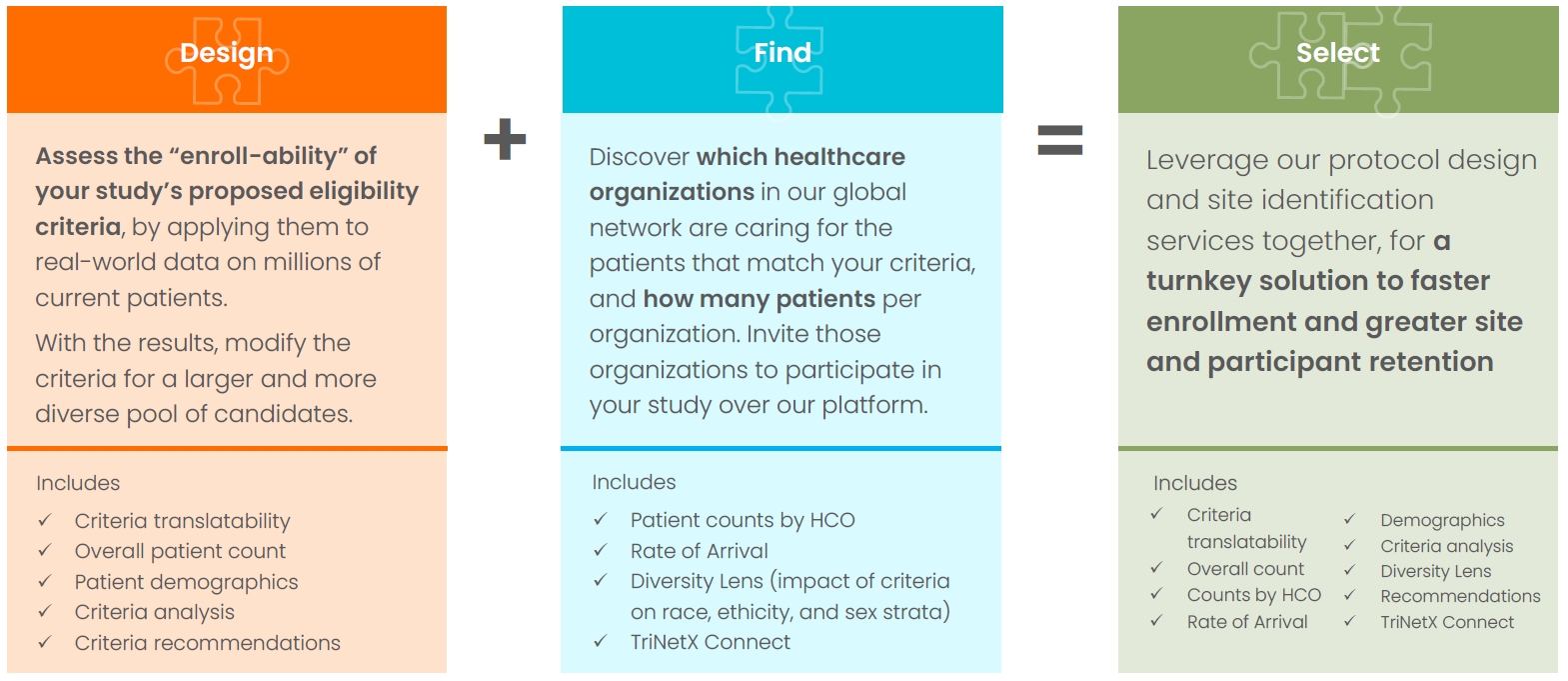
TriNetX Design, Find, and Select are a suite of consulting offerings for custom protocol design, feasibility, and site selection. The TriNetX Clinical Sciences team harnesses a deep knowledge of TriNetX data along with expert command of the TriNetX platform to deliver end-to-end protocol design and site selection using real-time real-world data. With TriNetX Design, our team will help you design and refine your protocol. TriNetX Find delivers a refined look into current and projected patient counts at more than 100 healthcare organization. TriNetX Select combines Design and Find for a comprehensive design, feasibility, and site selection experience.
Use Cases
-
- Criteria translatability
- Overall patient count
- Patient demographics
- Criteria analysis
- Criteria recommendations
- Patient counts by HCO
- Rate of arrival
- Diversity Lens
- Connect
- Leverage protocol design and site identification services together for a turnkey solution to faster enrollment and greater site and participant retention
Real-World Evidence Consulting
Led by Jeffrey Brown, PhD, and K. Arnold Chan, MD, ScD, our RWE consulting team brings decades of clinical, epidemiological, and regulatory experience to advance your goals. They’ve traveled every leg of the evidence generation journey, from protocol development and regulatory submission preparation to HEOR and market access projects. Our team works with you at each step of our collaboration, matching question to method and method to data, to ensure every deliverable is fit-for-purpose.
Use Cases
- Clinical development
- Pre-submission data generation
- Safety and other post-marketing requirements
- HEOR, Commercial, and Market Access studies

Publications & Abstracts
TriNetX data is research-grade and research-ready. No matter the analyses you conduct with TriNetX data, from RWE to HEOR, TriNetX is ready to work with you to prepare and publish abstracts and publications. TriNetX will work with you to conduct the analyses, write the full abstract and/or publication, and submit to the relevant journals and conferences.
Use Cases
- Cohort studies of patient outcomes
- Clinical support for laboratory findings
- Data source for predictive modeling
Data Set Curation
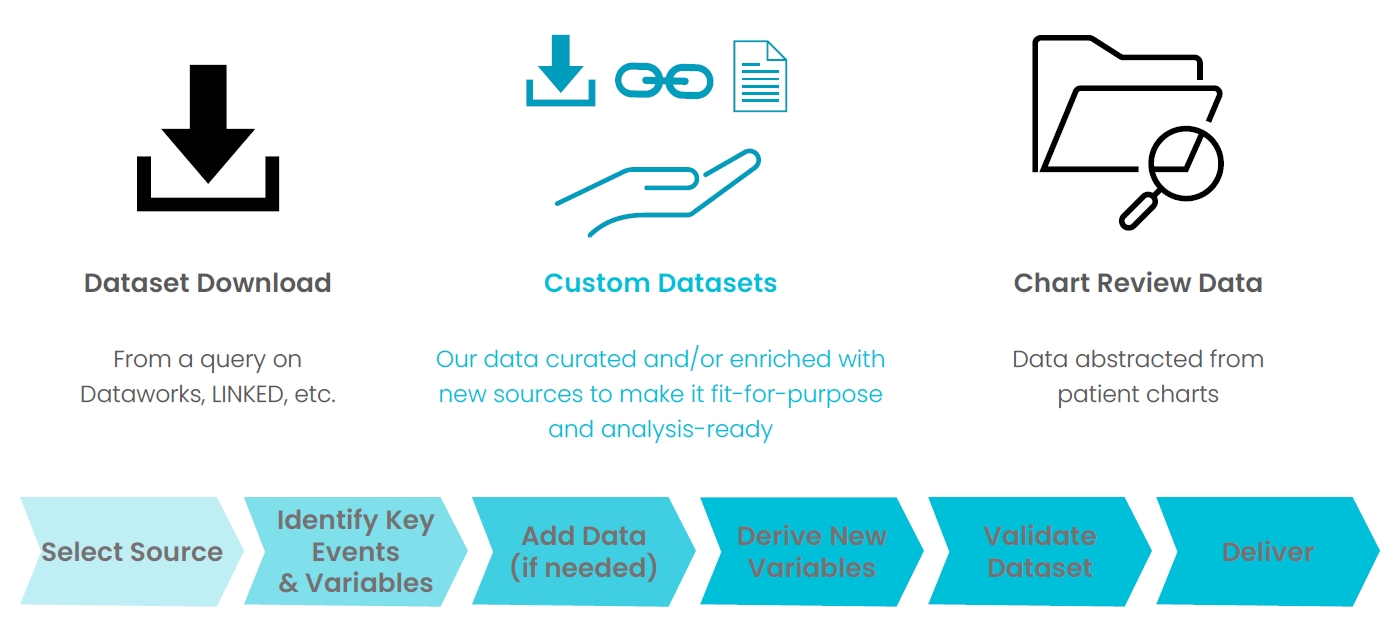
Finding the data you need for your research can be a complex exercise. Large data set purchases often contain data you don’t need; identifying appropriate inclusion/exclusion criteria can be time consuming and costly; there may be data sources well-suited to your needs that you haven’t identified. Allow TriNetX data and platform experts to take the burden of data curation off your plate. Our team will understand your data needs, build and iterate on a query across our global data network, and ensure that you are delivered one data set suited to your needs.
Use Cases
- Reduce need for large data set purchases and further curation of data
- Precision curation eliminates the need for repeated and iterative data set downloads
- Find symptomatic patients without an ICD-10 code
- Identify rare disease patients
Staff Augmentation
Imagine having a TriNetX employee sitting just a desk over to help answer your most complex clinical questions. Staff Augmentation makes that a reality for your team. TriNetX Staff Augmentation provides your team with a dedicated member of TriNetX Clinical Sciences to answer your specific RWD and research questions. TriNetX can schedule large-scale trainings according to your team’s schedule, and weekly meetings ensure that ongoing real-world evidence initiatives are meeting their milestones.
Use Cases
- Guidance for translating protocol inclusion and exclusion criteria
- Platform training for entire teams
- Tailored assistance for Advanced Analytics
- Support for defining cohorts