NON-US ONCOLOGY
Your Premier Source for Real-World Oncology Analyses in Europe
With TherapyMonitor, you can get comprehensive analyses derived from the latest longitudinal physician-supplied data on European oncology care. Collaborating with medical experts and pharmaceutical leaders, our European-based TriNetX Oncology team tailors report specifications for precise study objectives in oncological and rare-disease indications. Unearth complete, representative, and high-quality data, offering valuable insights into disease prevalence, treatment patterns, and market dynamics across the continent.

We help our partners show evidence of treatment value, fit for regulatory submission and publication, with timely, representative, longitudinal cancer care data in Europe.
What’s Inside TherapyMonitor
TherapyMonitor’s projects are based on carefully collected and organized data pertaining to patients over time, focusing on specific medical conditions or indications. This data includes structured information, such as demographics, medical history, and treatment regimens, and unstructured data, like clinical notes or imaging results. Additionally, the data is thoroughly curated to ensure accuracy and relevance to the specific project objectives.
With TherapyMonitor, all patient data is rendered fully anonymous, ensuring strict compliance with the most stringent regulations governing software and processes.
Precision Data Collection for Tailored Insights of Every Patient Journey
We collect thousands of variables per patient and treatment journey, from initial diagnoses to most recent encounters. Together with our clients and medical experts, we generate a fit-for-purpose questionnaire and database to match desired analyses.
- Comprehensive individual patient demographics and disease characteristics
- Highly granular diagnostics and cytogenetics data, therapy and drug-specific data including timestamps for all therapy events modifications
- Detailed information on outcomes, assessment method and progression and/or relapse definition according to clinical guidelines including caregiver definition. The outcomes are available for each separate treatment line
- Institutional characteristics and patient clinical pathway
PATIENT CHARACTERISTICS
Age, Performance, Height, Weight, Body Surface, Concomitant Disease, Symptoms, socio-demographic data, CCI-applicable.
DISEASE CHARACTERISTICS
Stage, Type, Biomarker Assessment, Cytogenetics, Metastases, Risk Assessment, Imaging Diagnostics, Laboratory values.
THERAPY
All therapy measures along clinical guidelines including Systemic Treatment, Surgery, High-Dose, Transplantation, CAR-T-cells, Consolidation, Supportive and Maintenance, Remission, and best-achieved results.
PROGRESSION & RELAPSE
Definition of Progression / Relapse based on Variables defined by Clinical Guidelines.
DRUG-SPECIFIC DATA
Dose, application way, Administrations per Cycle, Interval, Start- and Stop Date, Interruptions, modification.
THERAPY MODIFICATION
Dose, Administration per Substance, Interval, Interruptions, Intensification, De-escalation, Trial/Study Inclusion, Treatment Switch and Abort.
INSTITUTIONAL CHARACTERISTICS
Initiating vs Conducting Point of Care, by Care Sectors / Specialty.
Reflecting Real-World Patient Care in Europe
TherapyMonitor covers the EU5 and smaller European nations, providing a comprehensive view of cancer care across the continent. It encompasses a wide range of patient and disease data, treatment records, outcome assessments, and institutional information. Our data is of regulatory-grade quality, derived from representative samples of patients and healthcare institutions in each country included in our project scope of work.
Countries
including Germany, France, Spain, and United Kingdom
Patient Lives
Malignancies
hematological diseases and solid tumors
%
treated prevalence
Analysis precision: 95% CL / 05% CI
%
representative sample*
*based on regional epidemiological research and healthcare structure assessments, ensuring representation of treated prevalence across relevant healthcare sectors
Ongoing TherapyMonitor Projects
Stay Ahead with Quarterly Updates
TherapyMonitor provides regulatory-grade reports refreshed every 3 months, delivering up-to-date insights into patient data and outcomes. Leading healthcare innovation, our cutting-edge analyses shape critical decision-making processes.
TherapyMonitor analyses are regularly utilized for:
Health Technology Assessment & Epidemiology
Gain invaluable insights for health technology assessments and epidemiological studies supported by evidence-based data for informed decisions.
External Control Arms for Early & Accelerated Market Authorization
Access robust external control arms for accelerated market authorization, streamlining approval processes for novel therapies.
Current Insights in Therapy Algorithms, Patient- and Disease Characteristics, Care Pathway- and Outcomes Analyses
Dive into in-depth insights on therapy algorithms, patient and disease characteristics, care pathways, and outcomes analyses. Our expertly researched reports are often showcased at top medical conferences.
| INDICATION | COUNTRIES | SINCE |
|---|---|---|
| Breast Cancer | Germany | 2024 |
| Chronic Lymphocytic Leukemia (CLL) | Germany, Spain | 2021 |
| Multiple Myeloma (MM) | Germany, Spain | 2016 (Germany), 2023 (Spain) |
|
Urothelial Cancer |
Germany | 2023 |
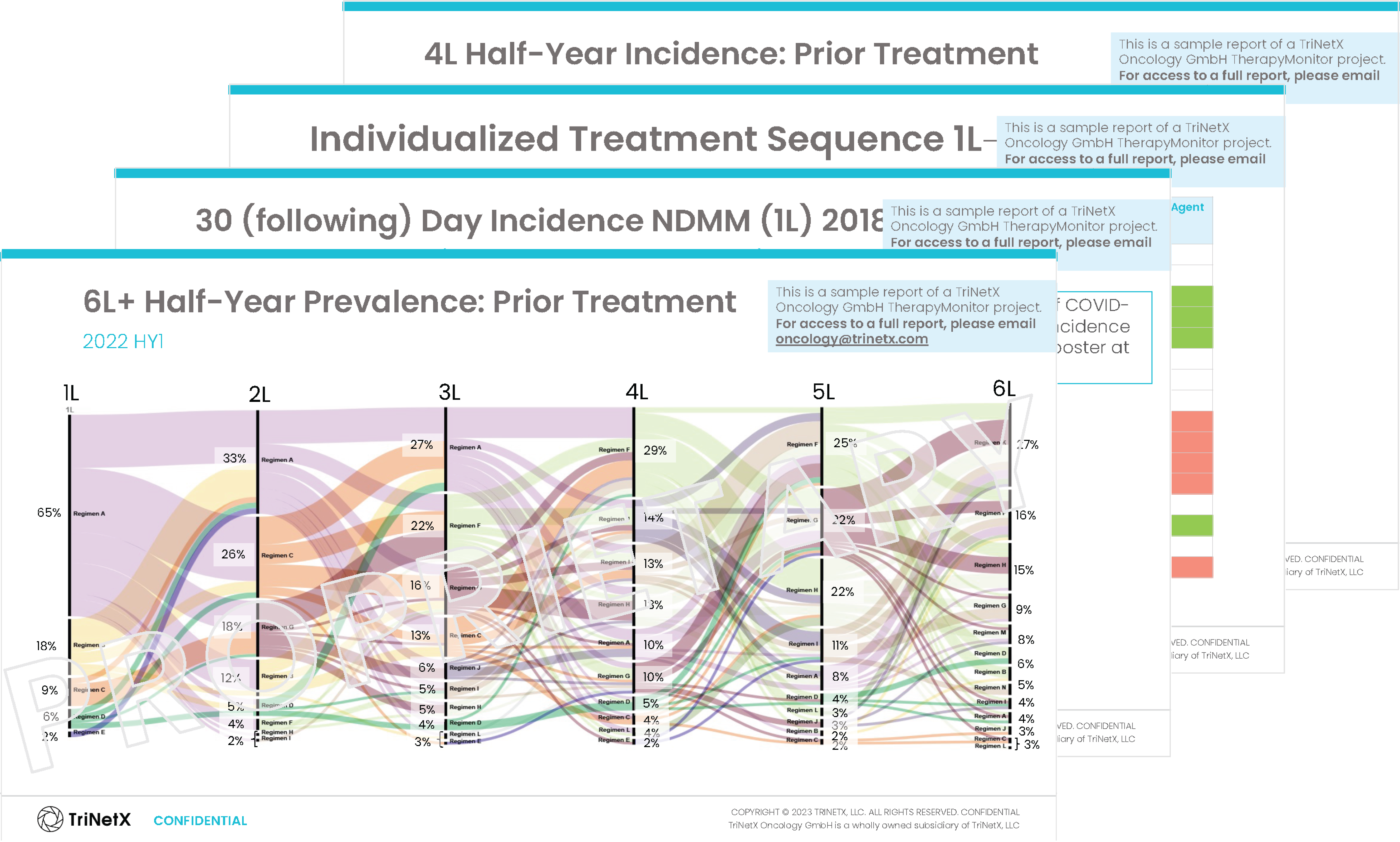
Download a Hematology TherapyMonitor Sample
TherapyMonitor
Explore a comprehensive analysis unveiling current disease prevalence, treatment patterns, and indication-specific market dynamics based on longitudinal, patient-level data. TherapyMonitor is scalable regarding indication and region, allows for fit-for-purpose research focused on specific target populations, and is based on fully anonymized data.
Inside you’ll discover insights on:
- Baseline/Demographics
- Current Therapy Status
- Initial Diagnosis
- Induction Therapy (for each line of treatment)
- Stem Cell Transplantation Measures (for each line of treatment)
- CAR-T Therapy Measures (for each line of treatment)
- Supportive Therapy and Adverse Event Prophylaxis (for each line of treatment)
- Best Achieved Outcomes/Response Assessments (for each line of treatment)
Going Beyond What’s Possible
As a global force, TriNetX continues to navigate uncharted territories with an unwavering commitment to revolutionizing the healthcare ecosystem.
Far beyond being a repository of information, TriNetX serves as a strategic partner in connecting health systems, providers, and researchers worldwide through our extensive real-world patient data that runs a mile wide and a mile deep.
From trial operations to evidence generation, we put you at the forefront of discovery.
Clinical Trial Design & Optimization
Data Sets & Analytics
Non-US Oncology
Real-World Evidence Generation
Pharmacovigilance & Drug Safety
TriNetX Oncology GmbH is a wholly owned subsidiary of TriNetX, LLC.
