DATASETS
& ANALYTICS
Access Fit-for-Purpose Real-World Datasets and Solutions.
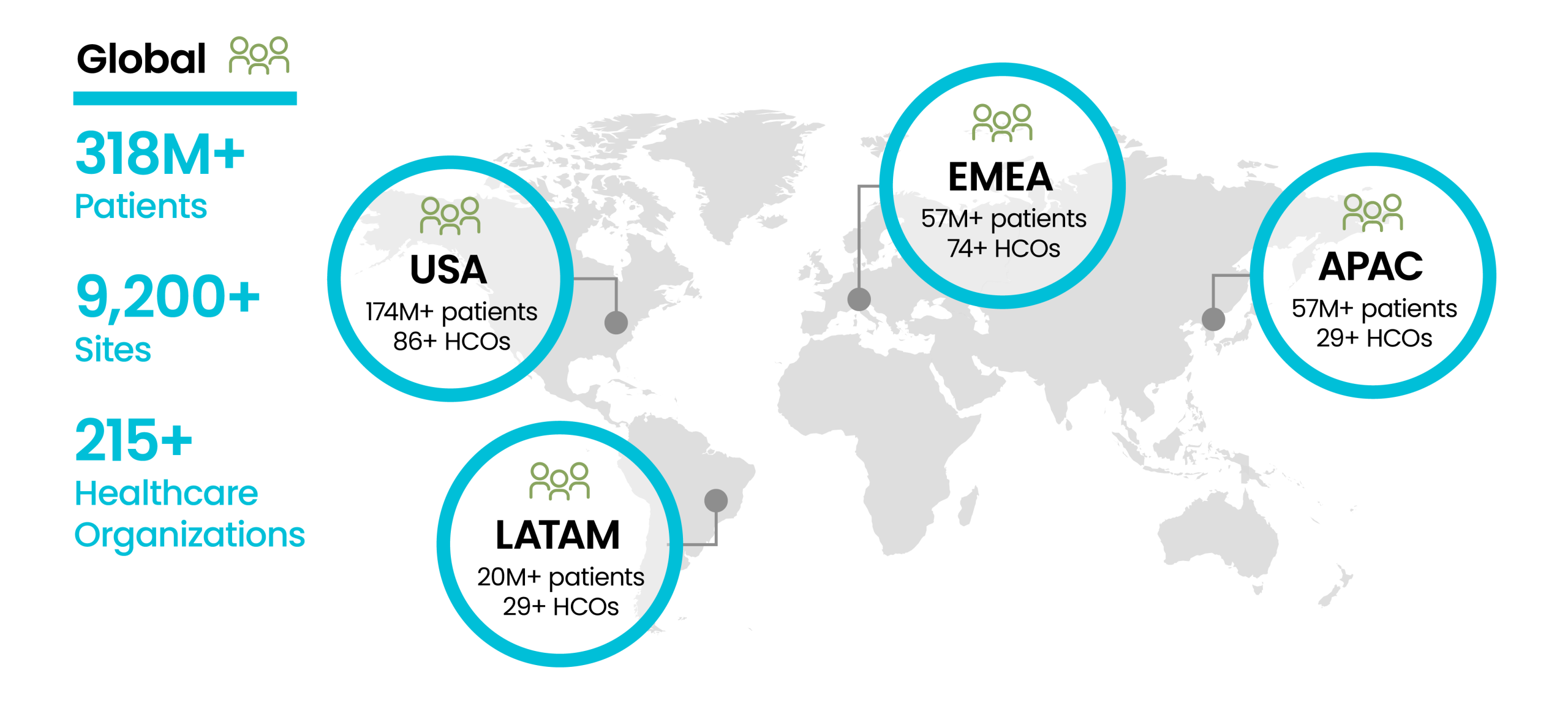
Access rich, harmonized datasets backed by a dedicated team of data scientists and researchers. Our extensive network provides comprehensive data on 117 million U.S. patients, refreshed every two to four weeks.

Downloadable, curated or linked datasets to power analysis. Plus, non-US, regulatory grade oncology registries to support market access and regulatory submissions.
Real-World Datasets
TriNetX delivers expertly curated real-world datasets that solve key challenges in data harmonization, access, and depth, enabling more informed research and decision-making through seamless data integration.
Dataset Services
Our dedicated team provides comprehensive data support – from assessment and extraction to data quality review and training – ensuring you get maximum value from your data.
Your questions deserve more than just answers—they deserve solutions, and we’re here to help you discover them.
TriNetX Datasets
- Dataworks: Downloadable, row-level, deidentified EHR patient data in a ready-to-use format for analysis on your platform. Fit-for-purpose datasets aligned to therapeutic areas, with enhancements available upon request.
- LATAM: Downloadable, deidentified data from private and philanthropic healthcare organizations. Dive into drugs, procedures, diagnosis, labs and oncology data from the LATAM region.
- Linked: Enriched EHR, medical claims, pharmacy claims, mortality, and SDoH data for detailed, longitudinal analysis.
- Custom Datasets: TriNetX downloadable EHR data combined with your third-party data to create unique datasets tailored to your needs, including new data from chart abstraction or clinical text search.
LICENSED DATASETS
Our largest single resource for data sets is built from EHR data enriched with labs and mortality data. Patients are from all 9 U.S. census divisions and three countries outside the U.S.
CURATED DATASETS
Curated data sets are designed to be immediately usable. They’re built upon a normalized, standards-based terminology that is an OMOP inspired, Native TriNetX Format, or the Sentinel Common Data Model, ensuring compatibility and ease of use. These research-ready data sets include calculated, derived variables like BMI, and new therapeutic-specific tables and facts purpose-built your analysis.
MULTIPLE MYELOMA
Unlike patient registries, this data set reflects observations and treatments that precede the cancer diagnosis, as well as the full spectrum of concurrent non-hematological care, for a holistic view of each de-identified patient.
PEDIATRIC DATA
Unlock the Power of Pediatric Data with TriNetX
Dive into one of the world’s most comprehensive, real-world data networks and enhance your research with high-quality, regulatory-grade datasets. Discover valuable insights into pediatric treatment patterns, safety signals, and health outcomes across the U.S. since 2010.
LINKED
De-identified U.S. patient data, including longitudinal histories from EHR, payer-sourced medical and pharmaceutical claims (“closed claims”) along with mortality data. This dataset ensures at least one pair of insurance coverage start and stop dates for each patient, enabling comprehensive care record analysis.
DATASET CURATION SERVICES
Navigating through the complexities of data acquisition for your research shouldn’t be a challenge. TriNetX is here to simplify the process. Let our data and platform experts handle the intricate task of curation. We’ll work closely with you to understand your specific needs and craft and refine queries across our global data network, ensuring you receive a tailored data set without the hassle.
TRINETX + AWS
AWS Data Exchange makes it easy to find, subscribe to, and use third-party data in the cloud. With AWS Data Exchange, customers can integrate TriNetX data and other third-party data sources into any of their company’s software via API requests without ETL. They can simply use their AWS credentials and AWS SDK to call APIs from dozens of data providers. All data is encrypted at rest and in transit, and the service is integrated with AWS identity and Access Management (IAM) to create fine-grained controls.
DATASET SERVICES
When you access TriNetX real-world datasets, you benefit from comprehensive support including:
- Data Domain and Data Element
- Feasibility Assessment
- Data Extraction, Design, and Planning
- Data Quality Review and Acceptance
- Training on Use of the Data
- Ongoing Customer Support
Why Choose TriNetX Real-World Datasets and Solutions
Comprehensive Coverage
- Research-ready data standardized to common terminologies
- 117 million U.S. patients with comprehensive longitudinal data
- Over 44 million tokenized patients linkable to third-party datasets
- Data refreshed every 2-4 weeks for maximum recency
Proven Impact
- Supported hundreds of peer-reviewed publications across multiple therapeutic areas
- Partnerships with leading healthcare organizations worldwide
- Validated through extensive use in regulatory submissions
- Led by internationally recognized experts in RWD/RWE
Flexible Access
- No long-term commitments required for accessing datasets
- Multiple delivery options
- Seamless integration capabilities with existing data sources
- Customization options for specific therapeutic areas
Unlocking Rare Disease Insights
Rare diseases affect millions, yet traditional research falls short. This report reveals how real-world data (RWD) is revolutionizing the field. Discover how strategic RWD applications, powered by the TriNetX LIVE™ platform, accelerate diagnosis, inform development, and close critical evidence gaps, reshaping solutions for underserved rare disease communities.
Submit your details to access the report, CSO Perspectives: Research Impact Report, and discover how partnering with TriNetX will enable you to advance rare disease research.

Access and Analyze Rich, Secure, Real-Time Data
TriNetX is a global network of healthcare organizations and life science companies, driving real-world research to accelerate the development of life-saving therapies.
From trial operations to evidence generation, we put you at the forefront of discovery.
Clinical Trial Design & Optimization
Datasets & Analytics
Non-US Oncology
Real-World Evidence Generation
Pharmacovigilance & Drug Safety