
Mar 14, 2024 | Blog, Published Research
Powered By TriNetX MIT and BIDMC’s study published by The Lancet introduces PRISM, a model for early pancreatic cancer risk, using a database of over 156 million de-identified US patients from TriNetX, LLC. PRISM accurately forecasts pancreatic cancer risk 18...
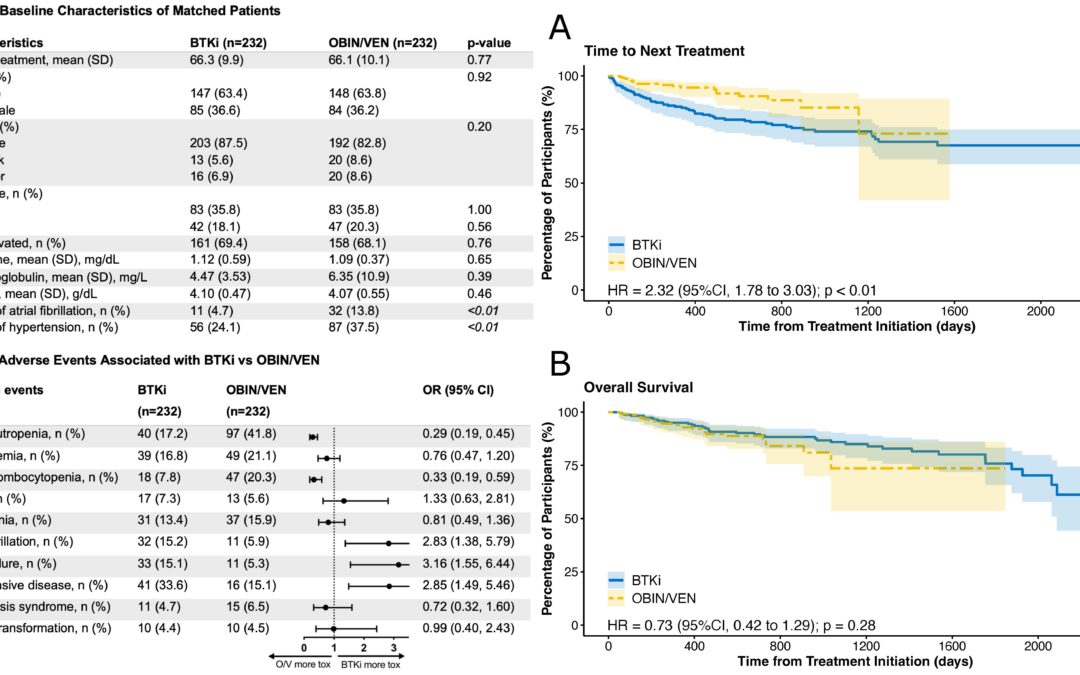
Jan 6, 2023 | Blog, Events, News, Published Research
In December, a contingent of our real-world data experts attended the 2022 Annual Meeting of the American Society of Hematology. Led by Chief Scientific Officer Jeffrey Brown and Senior Vice President, TriNetX Oncology GmbH Lenka Kellerman, our team spent their time...
Apr 13, 2019 | Published Research
This study was written by Manfred Stapff, MD, PhD, Chief Medical Officer at TriNetX, and Sarah Hilderbrand, MSc and published in the Journal of Clinical Hypertension describing how electronic medical records can be used to analyze and compare actual treatment...
Dec 15, 2018 | Published Research
This study published in the World Journal of Diabetes evaluates the effect on cardiovascular outcomes of sodium-glucose co-transporter-2 (SGLT2) inhibitors in a real world setting by analyzing electronic medical records.Manfred Paul Stapff, CMO, TriNetX Inc.,...
Nov 12, 2018 | Published Research
This scientific poster investigates the risk of Parkinson’s Disease (PD) in patients taking adrenergic drug treatments. Download the poster...
Nov 12, 2018 | Published Research
This scientific poster is comparing the risk of suicidality in adult versus youth patients treated with serotonin reuptake inhibitors—A real-world evidence-based approach. Download the poster...




