TriNetX LIVE™ Platform
Our web-based platform, TriNetX LIVE™, puts you at the center of our real-world data and evidence ecosystem. With an interface that’s as powerful as it is easy to use, you can build and analyze cohorts drawn from around the world in just minutes. When you’re ready to invite these patients into a study, connect with their healthcare providers with a few clicks.
Explore Networks
Platforms Features
TriNetX LIVE™ Regional Networks
Cohorts on TriNetX LIVE™ are always drawn from a network: a set of healthcare organizations united by geography, care specialty, or research mission. Our regional networks, described here, are the largest and most essential to clinical trial operations and real-world evidence generation.
Global
Spanning 21 countries in the Americas, EMEA, and APAC, our largest federated network harmonizes data from EHR enriched with lab values and genomic reports. Most contributing healthcare organizations refresh their data monthly.
Patients
197M
Healthcare Organizations
160
Countries
21 (Australia, Belgium, Brazil, Bulgaria, Estonia, France, Georgia, Germany, Ghana, Israel, Italy, Japan, Lithuania, Malaysia, Poland, Singapore, Spain, Taiwan, UAE, UK, US)
Supported Features
Advanced Analytics
TriNetX Connect
USA
Explore the freshest data on patients across the United States. Find the exact cohort you’re looking for among pediatric and adult populations in all nine US census divisions, from academic medical centers and community hospitals.
Patients
150M
Healthcare Organizations
79
US Census Divisions
9 of 9
Supported Features
Advanced Analytics
TriNetX Connect
EMEA
The quickest flights to Europe and the Middle East. Build and explore your cohort from encounter, diagnosis, medication, and procedure codes, all mapped to common terminologies like ICD-10-CM and LOINC.
Patients
36M
Healthcare Organizations
58
Countries
14 (Belgium, Bulgaria, Estonia, France, Georgia, Germany, Ghana, Israel, Italy, Lithuania, Poland, Spain, UAE, UK)
Supported Features
Advanced Analytics
TriNetX Connect
Latin America
Our Latin America Network represents the majority of all healthcare seekers in Brazil, enabling powerful population-level research.
Patients
8M
Healthcare Organizations
15
Data Partners
1
Countries
1 (Brazil)
Argentina, Chile, Colombia, and Mexico coming to the network soon
Supported Features
Advanced Analytics
TriNetX Connect
Data Set Download
Japan Claims
Optimize your trials for the world’s third largest pharmaceutical market with on-demand DPC (“Diagnosis Procedure Combination”) data, the basis for Japan’s national medical service reimbursement system for acute inpatient care.
Patients
42M
Healthcare Organizations
>460 acute care hospitals, including >200 cancer care centers
Countries
1 (Japan)
Supported Features
Advanced Analytics
USA & Asia-Pacific Spotlight
Broaden your reach with patient data sourced from renowned institutions in Australia, India, Malaysia, and Taiwan.
Patients
153M
Healthcare Organizations
88
Countries
6 (Australia, Japan, Malaysia, Singapore, Taiwan, US)
Supported Features
Advanced Analytics
TriNetX Connect
TriNetX LIVE™ Features
Neither coding nor medical terminology mastery are required to define and analyze your cohort of interest. Simple cohorts (e.g., all patients with a T2DM diagnosis in the last year) take only a minute to build. More complex cohorts, defined by a temporally ordered combination of observations and treatments, take only a little longer, thanks to a set of clear logical connectors users may drag and drop as needed.
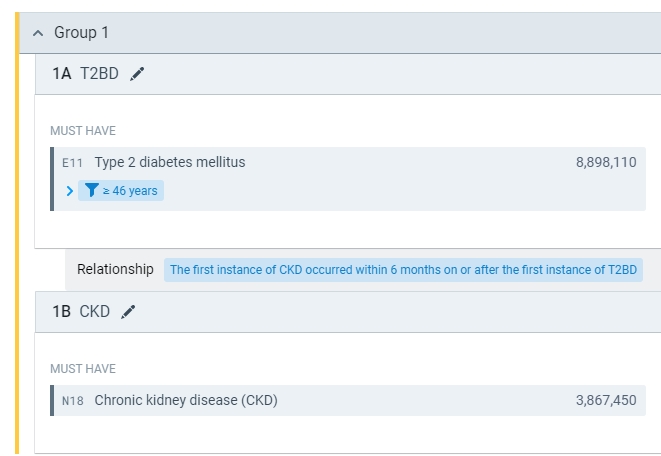
Query Builder
Define a cohort as precise and unique as your hypothesis. Query Builder lets you specify diagnoses, procedures, lab values, and more, standing in the temporal and logical relations you want.
Explore Cohort
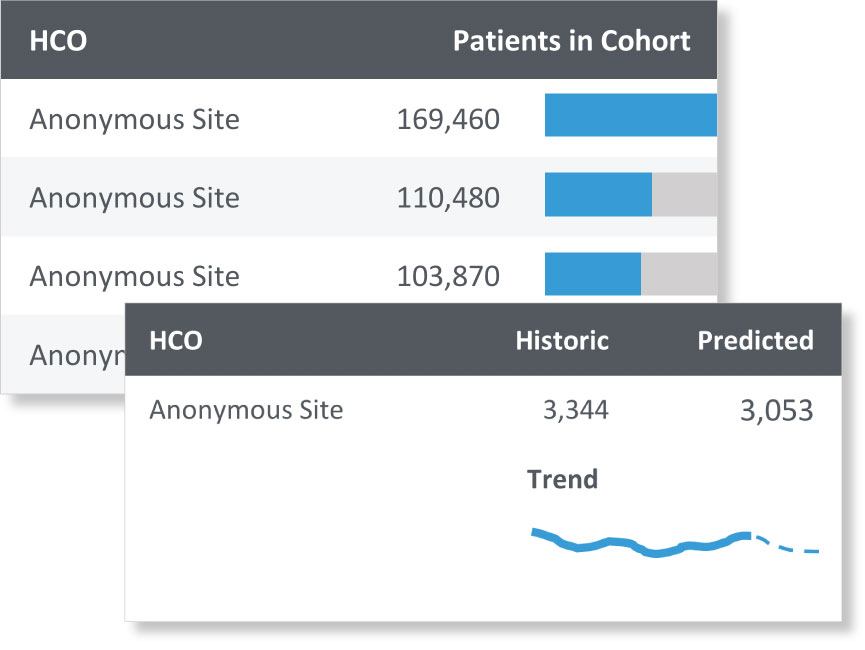
Instantly uncover a wealth of detail about your patients of interest, from the prevalence of medications prescribed to mean lab values.
Advanced Analytics
Analyze and compare outcomes, reveal treatment pathways, and more with statistically robust tools. Generate tables and graphs for presentation outside of our platform.
TriNetX Connect
Invite the healthcare organizations who provide our data to participate in your study. If your cohort includes patients drawn from their institution, they can re-identify them.
Introducing Diversity Lens
Better treatment for all starts with more inclusive trials.
Are your study criteria disqualifying one patient group more than another? By examining the impact of each criterion across demographic profiles, you can better ensure diverse enrollment from the start. That means more generalizable results, more opportunities for patients and providers, and more equity in the future of care.
See how we’re helping study designers turn their inclusion criteria into inclusive criteria with Diversity Lens.
Empowering Better Clinical Trial Designs with TriNetX LIVE™
Download a PDF overview of TriNetX LIVE™: A no-code, all-powerful interface for Clinical Trial Design & Optimization.
TriNetX LIVE™ is the world’s largest federated network of real-world data, harmonizing EHR, laboratory results, tumor registry information, and more into a common data model for on-demand cohort creation and analysis. The TriNetX LIVE™ platform aggregates recent and longitudinal observations on more than 300 million de-identified patients who have received or are receiving
care across the globe.


