 EVIDEX® Background Rate Analysis Tool
EVIDEX® Background Rate Analysis Tool
Ready to automate your background rate calculations?
The EVIDEX® Background Rate Analysis Tool provides an instant and cost-effective route to performing background rate analysis for initial signal assessment decisions when a signal is detected in a post-market source or an ongoing clinical study.
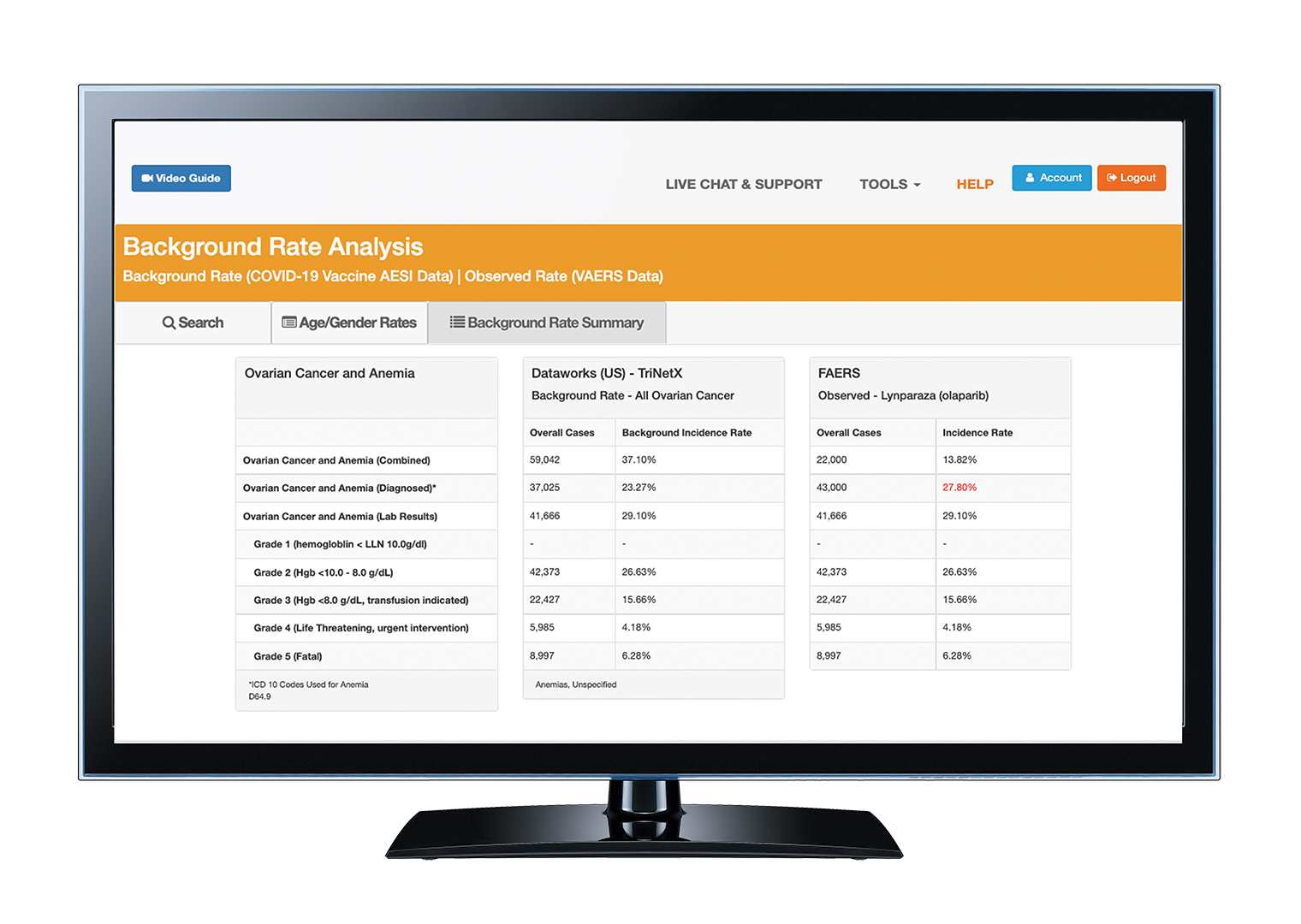
- The EVIDEX® Background Rate Analysis Tool allows users to calculate specific background incidence rates of adverse events for specific underlying conditions within a validated, GVP-IX compliant platform.
- Background rates are calculated using real-world patient data from TriNetX Dataworks (US) network covering over 50 healthcare organizations and 90 million patient lives.
On-demand background rate calculations within a few clicks.
Let us help guide you along a paramount, and expensive, step in the pharmacovigilance process of determining if a drug and adverse event pair is occurring at a rate that is higher or lower than the expected background incidence rate.
What’s Included: |
| Automation Users can calculate background incidence rates in minimal steps: select data source, underlying condition(s), and the preferred term – that simple! |
| Customization Background rates can be provided for adverse events within any indication or disease area(s) of interest. Contact our team to discuss which indications you’d like to explore. |
| Best-in-Class Customer Support Learn more about our highly responsive customer service. |
Ready to take the next step?
EVIDEX® is a registered trademark of Advera Health Analytics, Inc., which is a wholly owned subsidiary of TriNetX, LLC.
