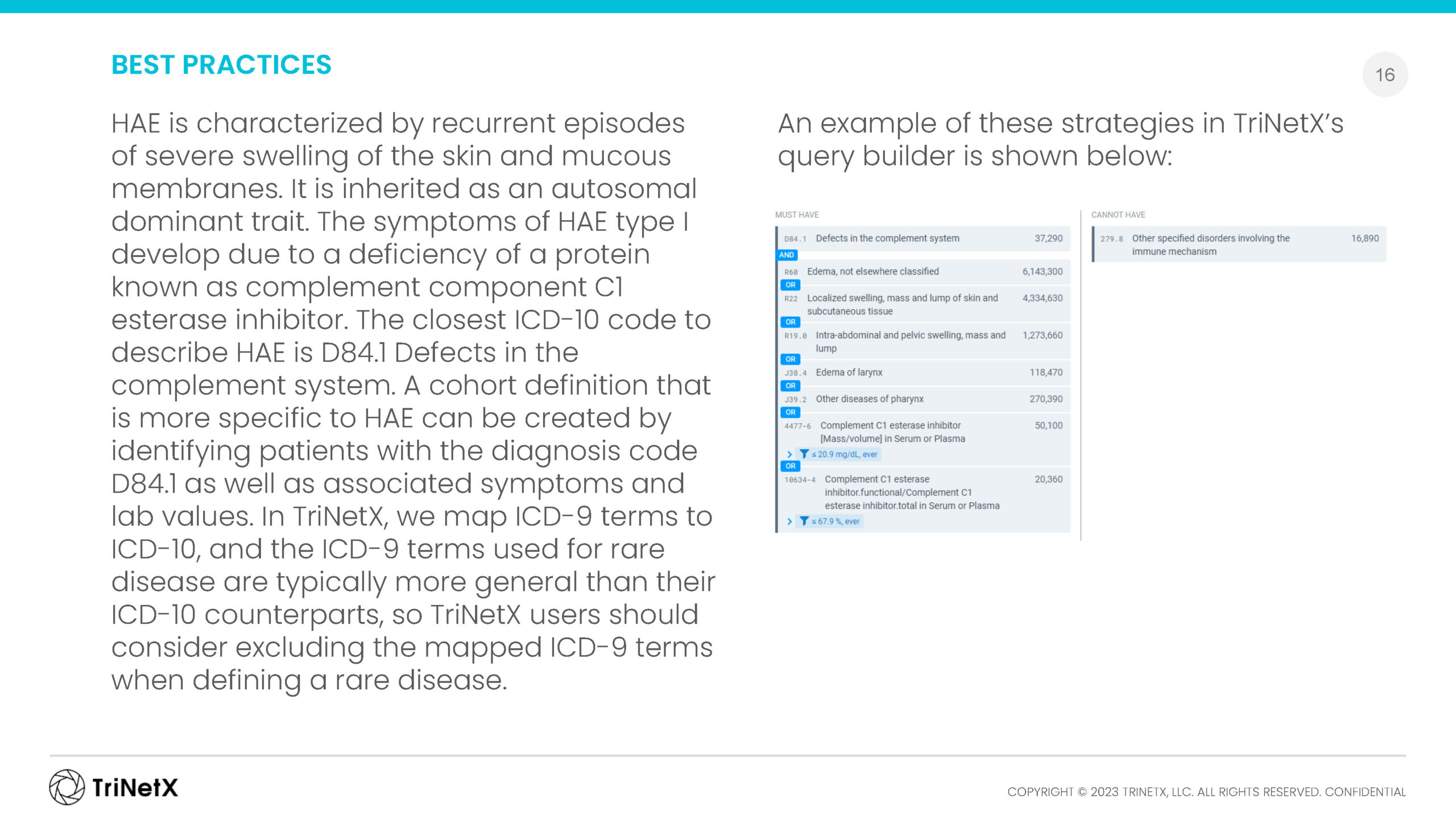
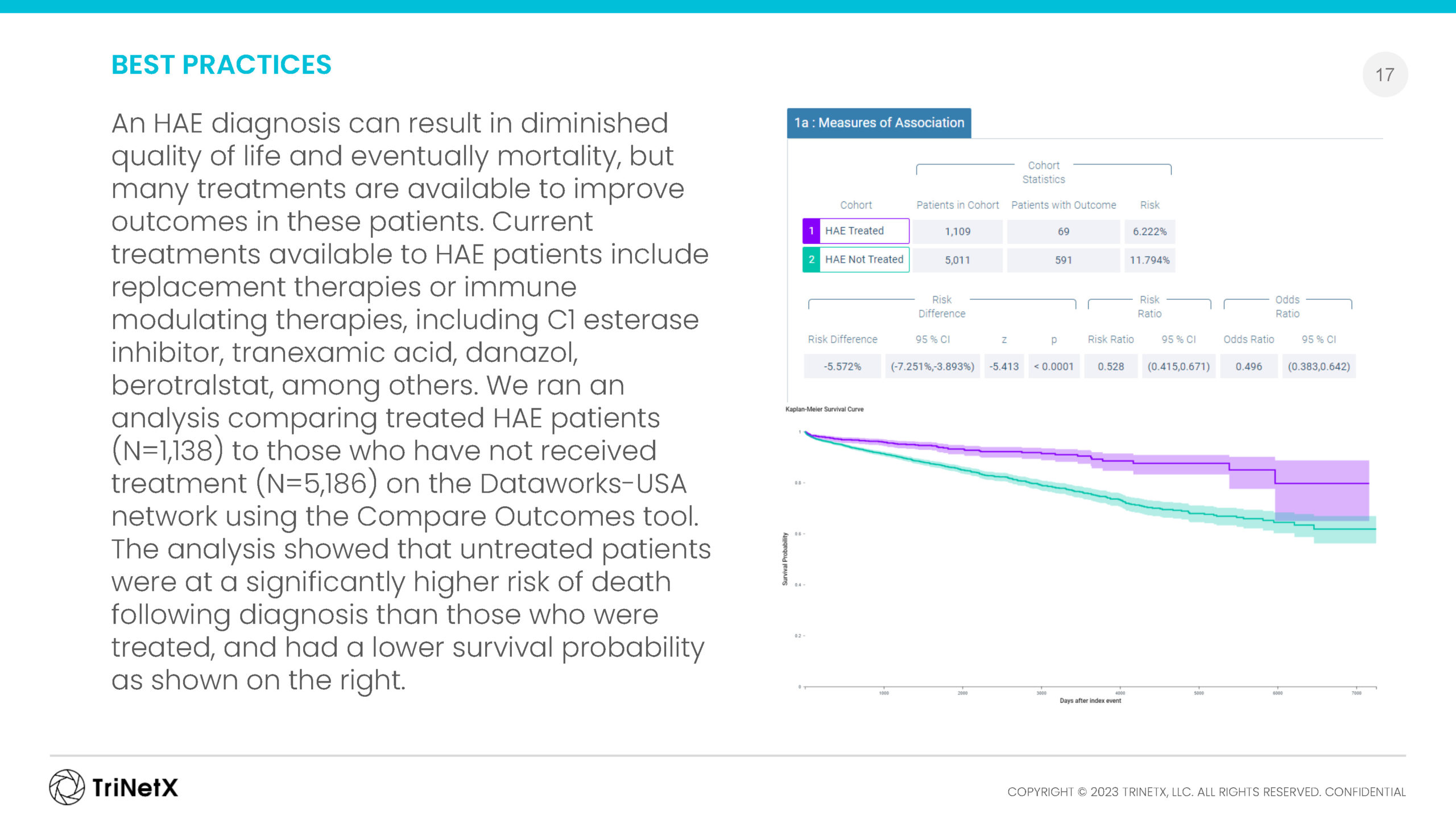
RARE DISEASE DATA
Today roughly 30 million patients in the U.S. live with a disorder characterized as rare by the FDA. Globally, hundreds of millions more fall into a similar category. In many cases, the course of their disease is poorly understood, and existing treatments – if they exist at all – are inadequate. Solving these problems starts with better research.
TriNetX is able to navigate you towards designing better rare disease protocols and clinical trials through:
Current & representative real-world data (RWD)
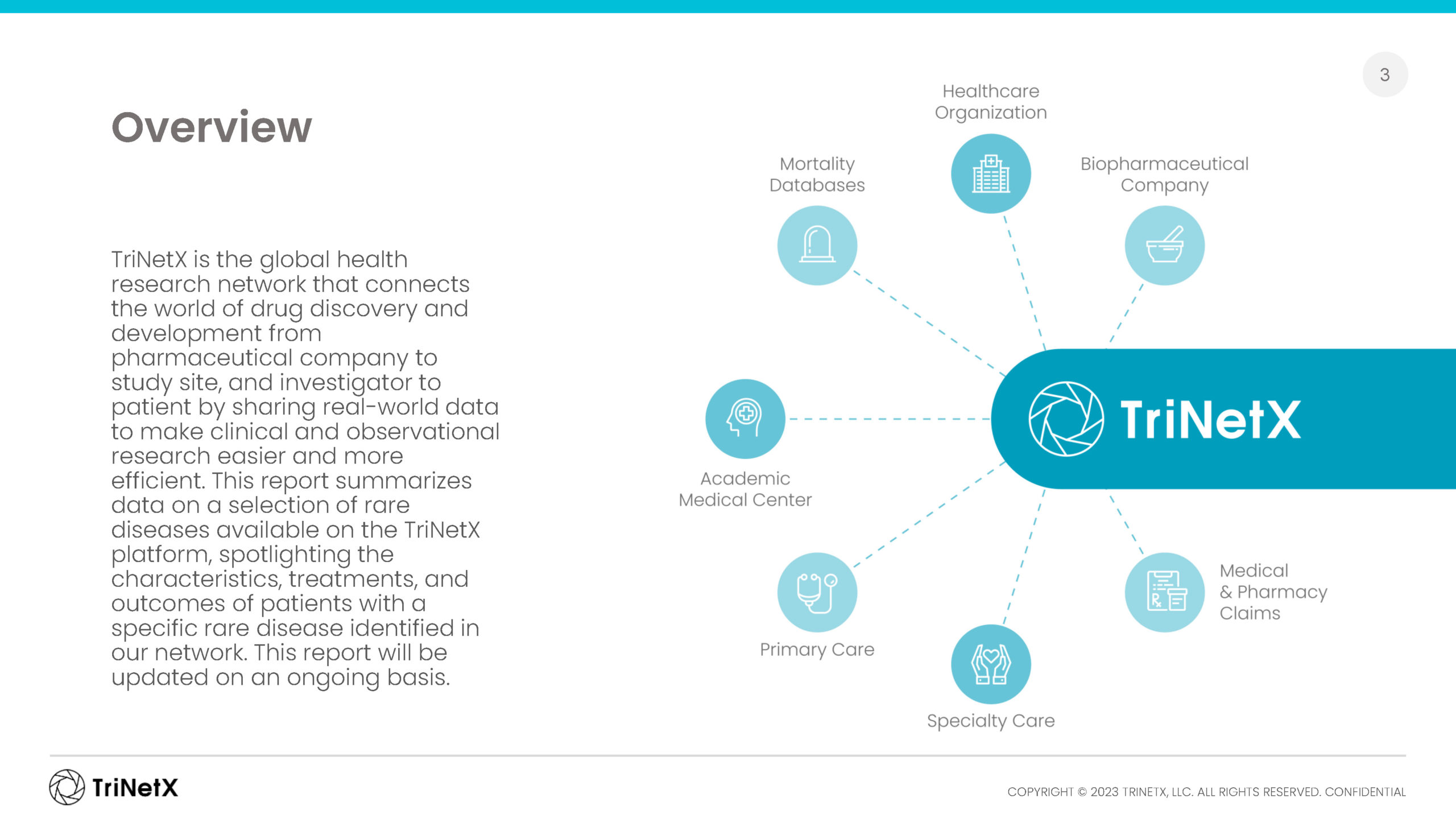
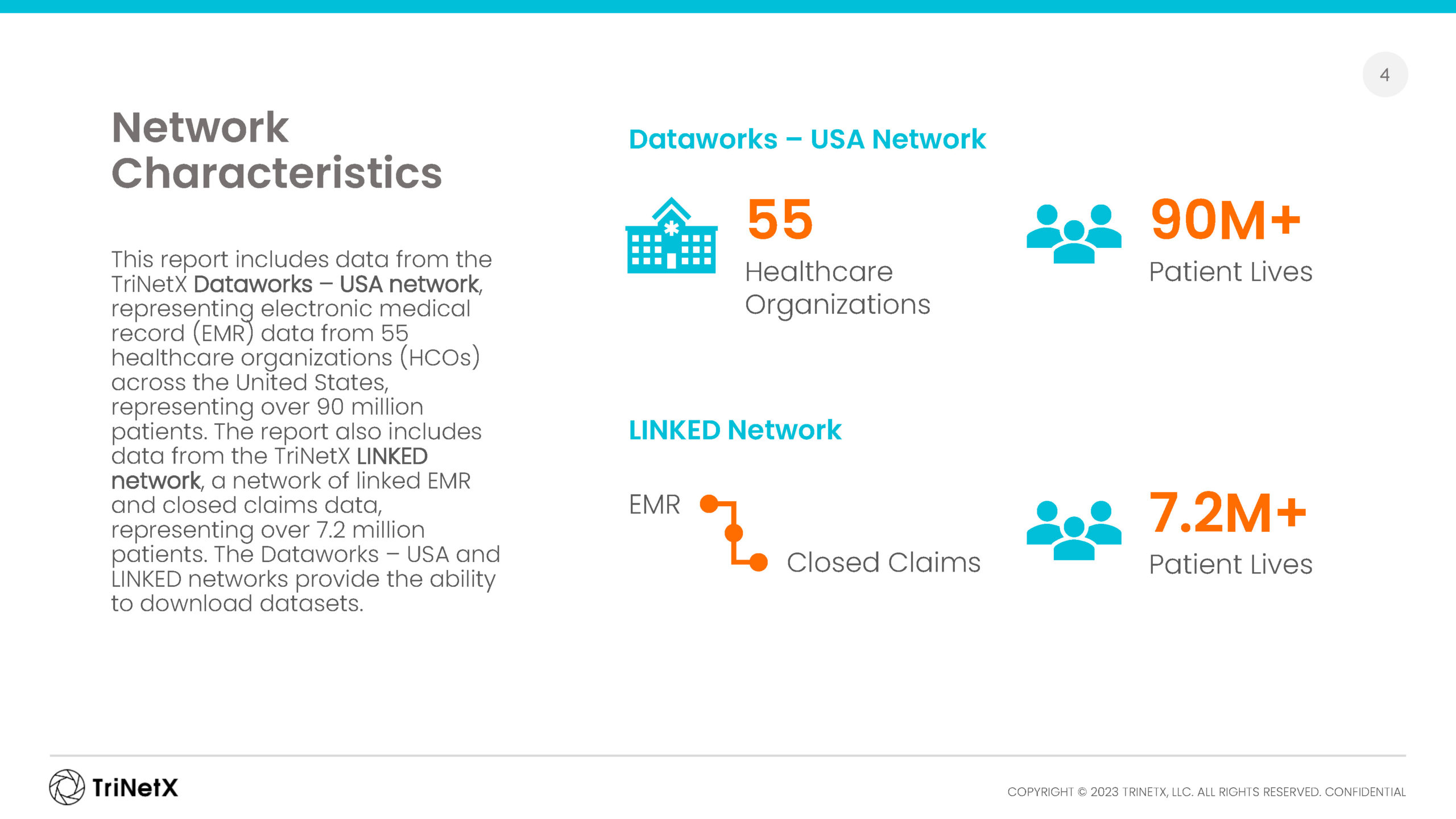
Our global network of healthcare organizations, together with data partners in Brazil, South Korea, and Japan, bring you tens of billions of clinical facts on more than 250 million patients around the world.
An intuitive RWD platform
Whether utilizing our data within our platform, through our data sets, or with our clinical sciences team, you can chart your course to novel patient insights. Depending on your project needs and scope, data may be either pseudonymized, de-identified, or anonymized.
Igniting actionable patient insights kindled from extensive patient populations
Our longitudinal data combines diagnostics, laboratory results, treatments and additional information like genomics and visit types allow for a more comprehensive view of relevant patient populations.
Better rare disease research starts with better data
Recognizing the rarest patients with the rarest needs
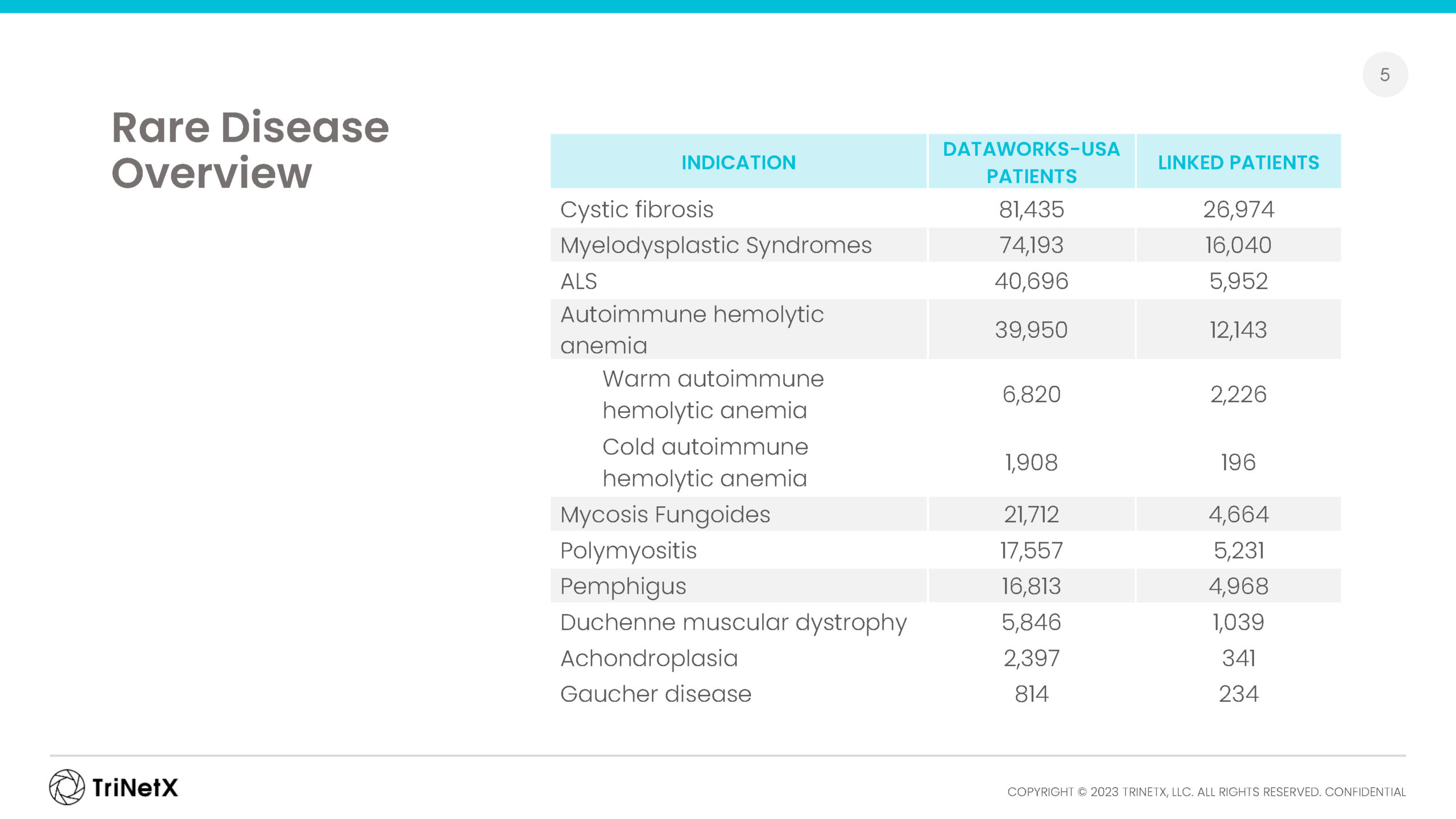
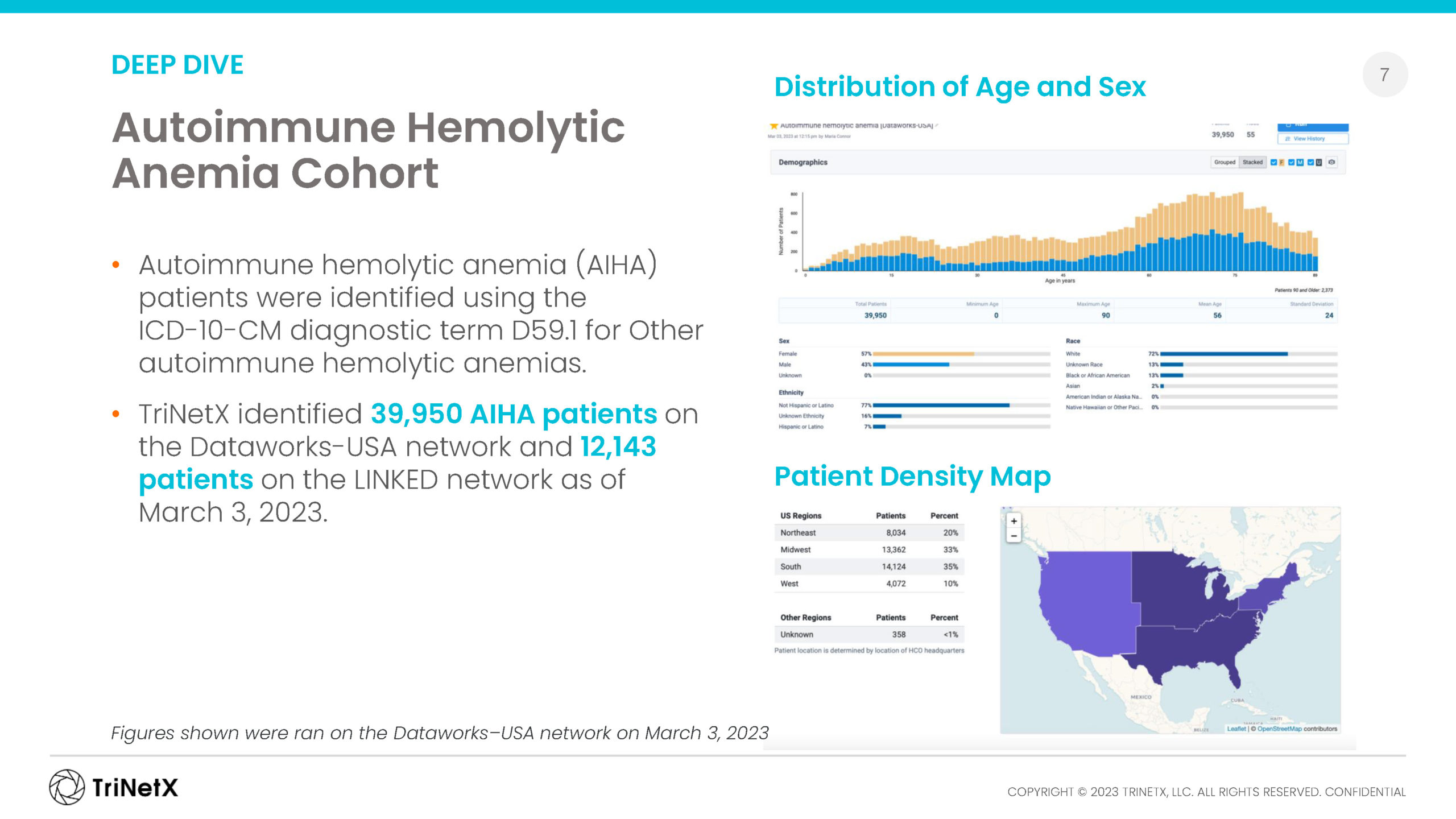
Browse our recent rare disease report, analyzing incidence, prevalence, and co-morbidities for a small cross-section of rare diseases represented in our regularly refreshed EMR data from across the U.S.
WATCH: Building Your Rare Disease Cohort with Real-World Data
“Rare” is relative. Today roughly 30 million patients in the U.S. live with a disorder characterized as rare by the FDA. Globally, hundreds of millions more fall into a similar category. In many cases, the course of their disease is poorly understood, and existing treatments – if they exist at all – are inadequate. Solving these problems starts with better research. And better research starts with the right cohort.
In this webinar, you’ll learn the best practices for defining a rare disease cohort from real-world data, using methods that go beyond a handful of diagnosis codes. Regardless of your current real-world data source, our clinical sciences team will show you how to conduct a “deep search” using laboratory, procedure, medication, and biomarker data to uncover patient records that do not always include a formal diagnosis. A member of our real-world evidence consulting team will then offer guidance on measuring the fitness of your cohort’s data for various tasks, from perfecting protocol designs to conducting retrospective research on effectiveness, safety, medication adherence, and more.
Rare Disease Research
See how experts are using our data and platform to advance their rare disease research
Let's start the conversation...
Connect with one of our rare disease experts to get answer to questions you have about TriNetX or discuss your rare disease project!