NON-US ONCOLOGY
Your Premier Analytics Source for Real-World Oncology Data in Europe
TriNetX offers non-US oncology real-world data services to help our partners show evidence of treatment value, fit for regulatory submission and publication, with timely, representative, longitudinal cancer care data in Europe.

Unearth representative, deidentified, and high-quality data, offering valuable insights into disease prevalence, treatment patterns, and market dynamics across the continent.
TherapyMonitor™: Non-US Oncology Analysis
Get comprehensive analyses derived from the latest longitudinal physician-supplied data on European oncology care. Collaborating with medical experts and pharmaceutical leaders, our European-based TriNetX Oncology team tailors report specifications for precise study objectives in oncological and rare-disease indications. Unearth complete, representative, and high-quality data, offering valuable insights into disease prevalence, treatment patterns, and market dynamics across the continent.
External Control Arms Design & Execution
TriNetX External Control Arms (ECAs) provide a proven methodology to create comparison cohorts without the high costs and lengthy timelines associated with traditional methods. TriNetX ECAs enable teams to generate control data that is representative and fit-for-purpose, enhancing clinical studies and regulatory submissions.
Epidemiological Support
TriNetX’s epidemiological services deliver evidence-based analysis and representative data so you can facilitate more informed decision-making, enhance your targeting and sampling efforts, and more deeply examine health outcomes. Backed by our strong record of successful HTA submissions, the breadth of our publication history, the depth of the TriNetX team’s experience, and the power of our data partnership with physicians and healthcare providers, you know you are in good hands.
Your questions deserve more than just answers—they deserve real-world solutions, and we’re here to help you discover them.
Exclusive Research & Data Collection Methodology. Premium Data Analysis & Service.
Tackle the challenges of oncology research in Europe with TriNetX.
Faced with fragmented data and limited patient access, researchers turn to TriNetX for comprehensive real-world data, enabling them to analyze treatment patterns and patient populations efficiently. Enhance your study with TriNetX external control arms, streamlining the creation of comparison cohorts while bypassing the lengthy timelines of traditional methods. Then with additional support from TriNetX epidemiological services, ensure your data is representative and ready for regulatory submission.
Together, these solutions help achieve improved clinical trial feasibility and better outcomes in oncology care.
What’s Inside TriNetX TherapyMonitor™ for Clinical Cancer Research
Download a PDF overview of TriNetX TherapyMonitor™.
TriNetX TherapyMonitor™ projects are based on carefully collected and organized data pertaining to patients over time, focusing on specific medical conditions or indications. This data includes structured information, such as demographics, medical history, and treatment regimens, and unstructured data, like clinical notes or imaging results. Additionally, the data is thoroughly curated to ensure accuracy and relevance to the specific project objectives.
With TriNetX TherapyMonitor™, all patient data is rendered fully anonymous, ensuring strict compliance with the most stringent regulations governing software and processes.
Ongoing TriNetX TherapyMonitor™ Projects
Accelerate Your European Oncology Insights
TriNetX TherapyMonitor™ delivers oncology insights in 6-7 months versus the typical 1+ year timeline, backed by an extensive provider network and proven success in regulatory submissions across EU5 markets.
Our epidemiologists tailor fit-for-purpose data samples aligned with your target population, leveraging deep relationships with physicians and institutions to ensure representative, submission-ready evidence.
TriNetX TherapyMonitor™ analyses are regularly utilized for:
- Market Access Strategy & Evidence Generation
- Treatment Sequencing & Regimen Distribution
- Patient Population Identification
- Standard of Care Analysis by Region
- Real-World Evidence for Regulatory Submissions
- Clinical Trial Feasibility & Planning
| INDICATION | CURRENT COUNTRIES |
|---|---|
| Chronic Lymphocytic Leukemia (CLL) – Second Line Therapy (2L+) |
Germany |
| Diffuse Large B-cell Lymphoma (DLBCL) | Germany |
| Estrogen receptor-positive (ER+), HER2-negative (HER2-) Breast Cancer | Germany |
| Newly diagnosed multiple myeloma (NDMM) Relapsed/refractory multiple myeloma (RRMM) |
Germany |
| Renal Cell Carcinoma (RCC) | Germany |
| Urothelial Cancer | Germany |
TriNetX Contact Information for Physicians in Europe
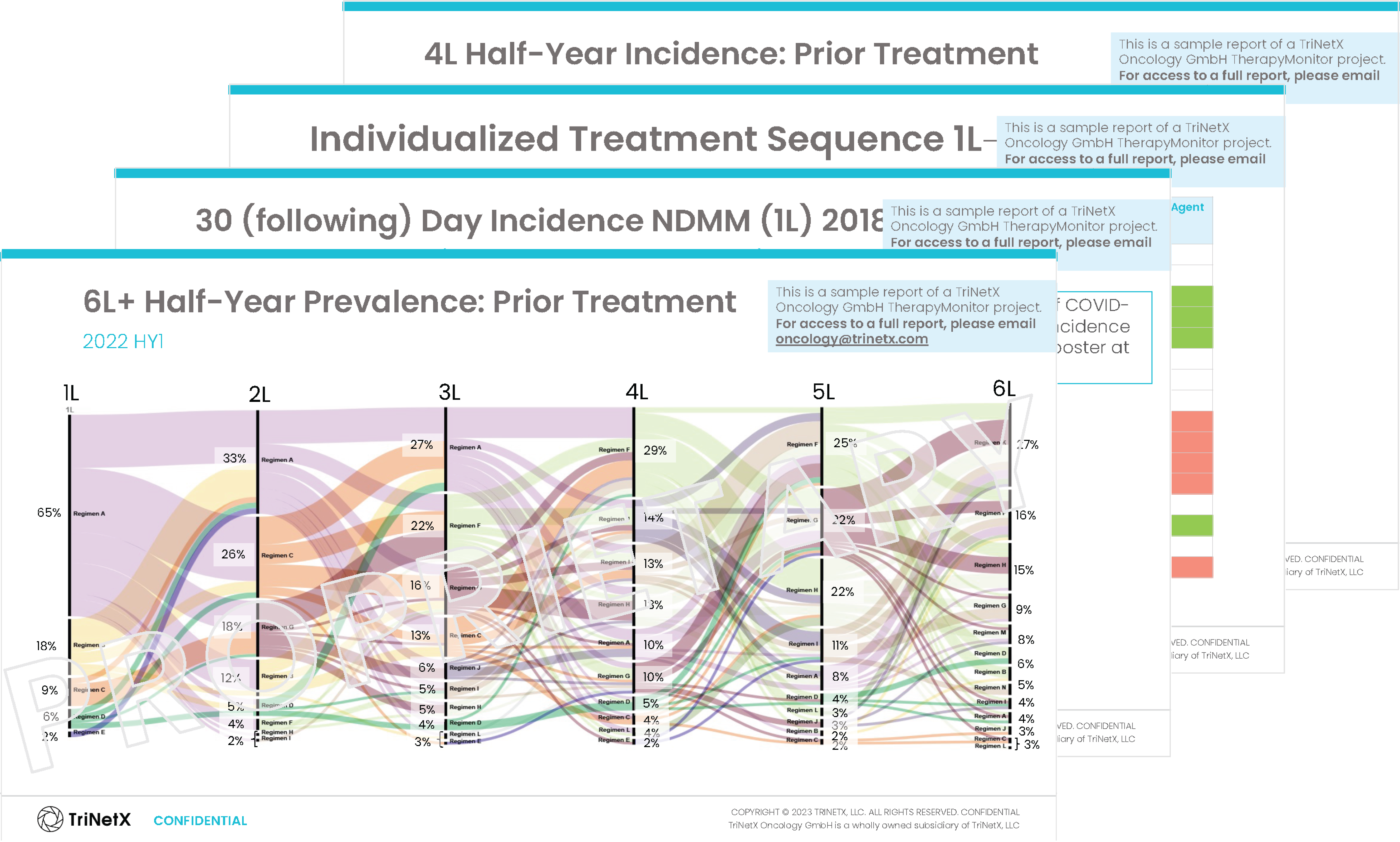
Download a TriNetX TherapyMonitor™ Sample for
Multiple Myeloma
TriNetX TherapyMonitor™
Reports are customized for your specific research needs, with TriNetX experts available to provide additional analysis and personalized insights.
This Multiple Myeloma example demonstrates our reporting capabilities. Metrics and analyses are tailored to your indication and objectives.
Inside you’ll discover insights on:
- Baseline/Demographics
- Current Therapy Status
- Initial Diagnosis
- Induction Therapy (for each line of treatment)
- Stem Cell Transplantation Measures (for each line of treatment)
- CAR-T Therapy Measures (for each line of treatment)
- Supportive Therapy and Adverse Event Prophylaxis (for each line of treatment)
- Best Achieved Outcomes/Response Assessments (for each line of treatment)
Explore Real-World Oncology Research in Europe
See how TriNetX is transforming oncology research across Europe. Discover how real-world data can accelerate clinical research and advance patient care.
Access and Analyze Rich, Secure, Real-Time Data
TriNetX is a global network of healthcare organizations and life science companies, driving real-world research to accelerate the development of life-saving therapies.
From trial operations to evidence generation, we put you at the forefront of discovery.
Clinical Trial Design & Optimization
Datasets & Analytics
Non-US Oncology
Real-World Evidence Generation
Pharmacovigilance & Drug Safety
TriNetX Oncology GmbH is a wholly owned subsidiary of TriNetX, LLC.
TriNetX Contact Information for Physicians in Europe:
Tel: +49 761 – 38 39 94 – 0 | Fax: +49 761 – 20 25 117 | oncology@trinetx.com
Complete the form below to access a sample TriNetX Oncology GmbH TherapyMonitor Report:
"*" indicates required fields

