SCOPE Europe: Summit for Clinical Ops Executives
Tuesday 14th – Wednesday 15th October 2025
InterContinental, Barcelona (Fira Center), Spain
Book a Meeting
Are you looking to reduce time-to-market, optimize trial designs, and enhance patient recruitment?
Meet with us at SCOPE Europe to learn how
Our team of experts will be onsite at SCOPE Europe showcasing how TriNetX’s real-world data solutions can accelerate and optimize your clinical trials. Stop by booth #1.
Find our Booth Location
Accelerate and Optimize your Clinical Trials
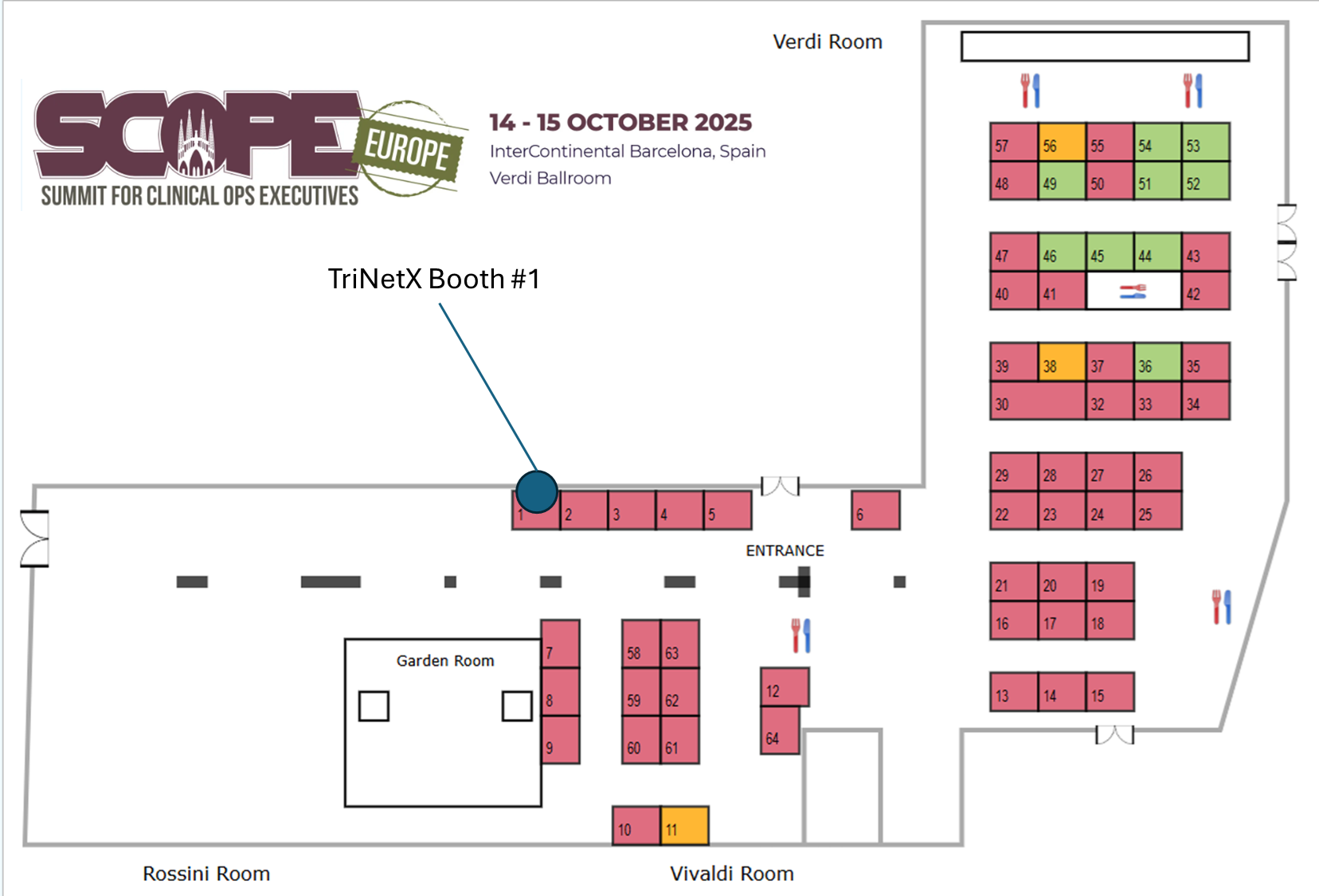
Find us at Booth #1
Meet with our RWD Experts at booth 1 throughout the conference or schedule a live demo of the TriNetX LIVE™ Platform to help accelerate and optimize your clinical trials.
Connect with TriNetX Clinical Trial Experts
Join us at SCOPE EU to explore how TriNetX can become your premier partner in the next generation of clinical trial design and optimization for improved clinical operations. Browse speaking engagements from the curated conference agenda below.
Podium Presentation
Date: Wednesday 15 October 2025 at 9:15am (25 Minutes)
Track C4: Clinical Data Strategy and AI Innovation
Title: Unlocking Clinical Expertise: Empowering Efficient EHR Search with Natural Language Queries
Abstract: Discover how natural language queries powered by LLMs are transforming EHR and RWD searches. By enabling intuitive, conversational access to clinical data, these tools improve efficiency, accuracy, and usability. This session explores challenges with traditional coding-based searches, real-world case studies, and future directions, highlighting how clinicians can retrieve insights faster and improve patient care.
Presenter: Justin North, Vice President, Product Management, TriNetX
By putting patients at the forefront of site selection, we are promoting a better patient experience by targeting sites the patient already visits frequently as well as decreasing the overall timelines of patient recruitment at the site.
Take the next step in accelerating and optimizing your clinical trials
Explore the possibilities by speaking with our RWD experts.
Not going to SCOPE EU?
Take a look at our forthcoming events and take your seat with TriNetX. Or view our webinars, available on-demand and across a wide spectrum of topics.
Access and Analyze Rich, Secure, Real-Time Data
TriNetX is a global network of healthcare organizations and life science companies, driving real-world research to accelerate the development of life-saving therapies.
From trial operations to evidence generation, we put you at the forefront of discovery.
Clinical Trial Design & Optimization
Datasets & Analytics
Non-US Oncology
Real-World Evidence Generation
Pharmacovigilance & Drug Safety
Events with TriNetX
Please complete the form below to request a meeting with the TriNetX team, and learn how you could become part of the connections improving human health across the world.
