ESMO Congress 2024
September 13-17, 2024 | Barcelona, Spain
Uncover Solutions to Even the Most Difficult European Oncology Research Challenges
Book a Meeting
Expert Experience
Resources
Expanding European Oncology Research Possibilities.
At TriNetX, we are revolutionizing European oncology research through our commitment to providing unparalleled access to real-world data insights. Our cutting-edge platform, extensive data analytics, and a team of seasoned experts empower researchers to unlock the transformative potential of real-world evidence.
What truly sets us apart is our dedication to delivering comprehensive analyses that illuminate current disease prevalence, treatment patterns, and market dynamics. Our US-based data leverages longitudinal patient-level data derived from electronic health records, with the capability to link to claims data, mortality information, and other data sources. Outside the US, our team of oncology experts leverages longitudinal, indication-specific patient-level data to offer unparalleled insights into the European oncology healthcare landscape. As the sole provider of comprehensive analyses from physician-supplied data on oncology care in Europe, we provide researchers with unique opportunities to delve deeper into their studies and drive impactful outcomes for patients and healthcare systems alike.
Meet the TriNetX Team at ESMO 2024
Join us at ESMO 2024 to explore how TriNetX can become your premier source for real-world oncology analyses in Europe. Book time with our subject-matter experts on-site at this year’s ESMO Congress. Using timely, representative, longitudinal cancer care data in Europe, we help oncology researchers demonstrate treatment value, fit for regulatory submissions and publications.

Andreas Weber
Senior Vice-President, TriNetX Oncology
Andreas brings a wealth of experience to the TriNetX team, following a distinguished 30-year career in the life sciences industry. Most recently, he served as the CEO of EvidentIQ, a leading provider of technology and data science solutions for clinical research. His career includes senior management roles at ERT/BioClinica and Oracle Health Sciences, where he built a 17-year sales and business development legacy.
As a senior executive with a proven track record in management and leadership, Andreas is known for his deep understanding of life sciences, spanning the entire industry continuum. His expertise encompasses pharmacovigilance, drug safety, regulatory affairs, and end-to-end eClinical processes, with a strong focus on business process optimization, outsourcing, and information technology.
Andreas’s career reflects a commitment to advancing the life sciences industry through strategic innovation and effective leadership, making him a valuable asset to TriNetX and a respected figure in European oncology research.

Markus Rückert, PhD
Medical Director
Markus is a seasoned biopharmaceutical expert specializing in oncology and rare diseases, boasting over 20 years of extensive experience in leadership positions across renowned organizations such as Ipsen Pharma, CSL Vifor, and MorphoSys, among others. Proficient in clinical development and Medical Affairs, as well as experimental and real-world evidence generation, Markus has successfully navigated various stages of drug development—from discovery to market launch—with a keen focus on advancing oncological and rare disease therapies for tangible patient benefits. Having achieved significant milestones in oncology and rare disease therapeutics, Markus now channels his diverse expertise towards broader pursuits in epidemiology, health service research, and the real-world impact of oncological treatments. Holding a PhD in biochemistry and pharmacology, Markus has contributed academically to esteemed institutions such as the University of Würzburg, Germany, and the Karolinska Institutet, Stockholm, Sweden.
The European-based TriNetX oncology team exists to help you achieve your goals, no matter your leg of the evidence generation journey. Let us help you move forward with the speed and confidence that only decades of clinical and regulatory experience can provide.
Pre-Book Meetings with TriNetX
Join us at ESMO 2024 to explore how TriNetX can enhance your research capabilities. Meet our subject-matter experts to discuss how our real-world data solutions can support your specific research needs and contribute to advancing oncology research in Europe.
2024 ESMO European Oncology Resources
Explore the latest innovations in European oncology research with our collection of 2024 ESMO posters, featuring insights from TriNetX experts, TherapyMonitor reports, and real-world data networks. Our resources highlight transformative advancements in real-world data analytics, offering a comprehensive look at pioneering research and emerging trends in European oncology.
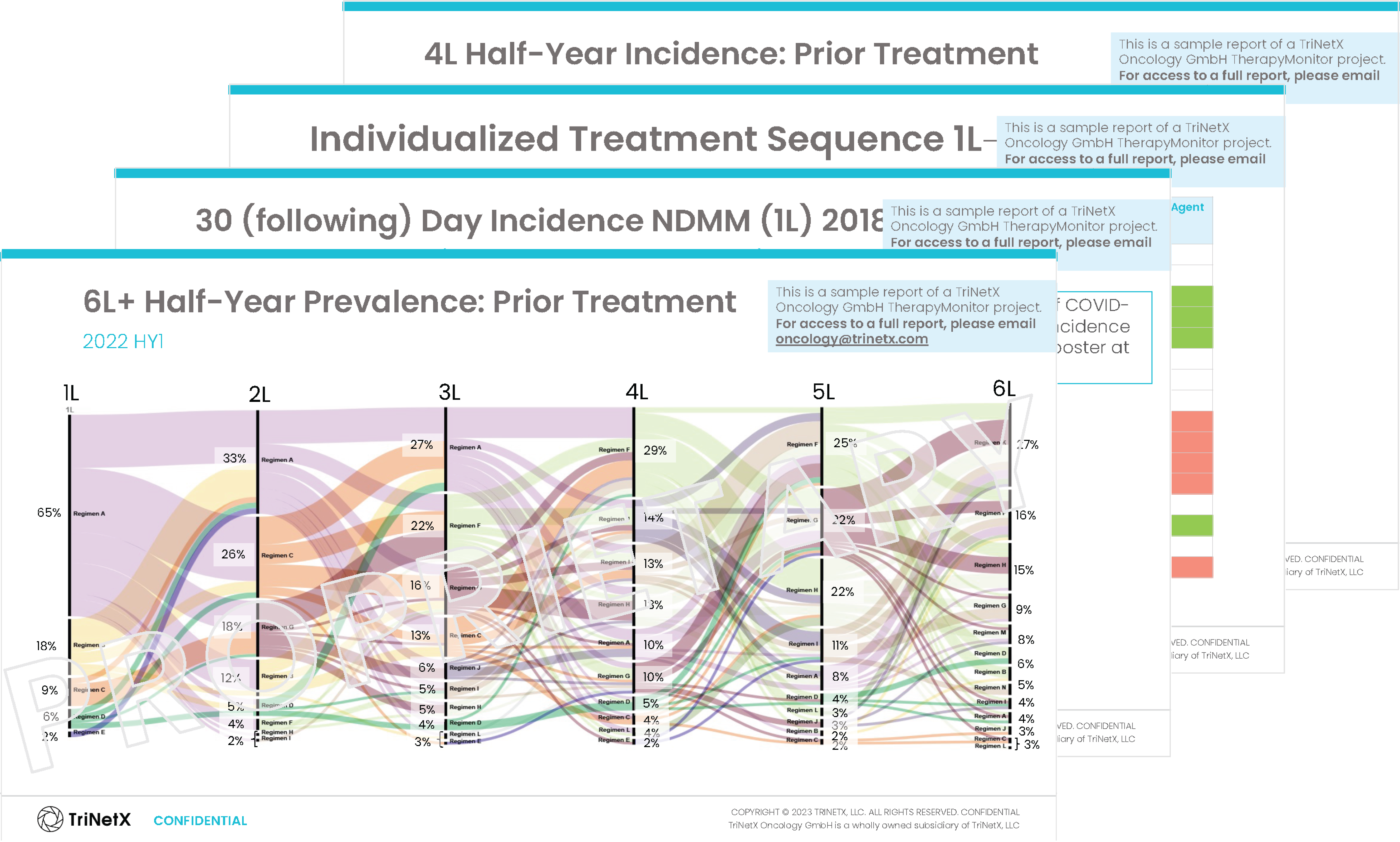
TherapyMonitor
Explore a comprehensive analysis unveiling current disease prevalence, treatment patterns, and indication-specific market dynamics based on longitudinal, patient-level data. TherapyMonitor is scalable regarding indication and region, allows for fit-for-purpose research focused on specific target populations, and is based on fully anonymized data.
Inside you’ll discover insights on:
- Baseline/Demographics
- Current Therapy Status
- Initial Diagnosis
- Induction Therapy (for each line of treatment)
- Stem Cell Transplantation Measures (for each line of treatment)
- CAR-T Therapy Measures (for each line of treatment)
- Supportive Therapy and Adverse Event Prophylaxis (for each line of treatment)
- Best Achieved Outcomes/Response Assessments (for each line of treatment)
The Future of Oncology Research in Europe: Integrating Real-World Evidence into Joint Clinical Assessments
The implementation of JCAs brings several key changes and is expected to impact how pharmaceutical companies develop, present, and seek market access for new drugs and health technologies throughout the EU. TriNetX RWD services are helping to accelerate oncology and rare disease data analysis to address JCA requirements.
Harnessing Real-World Data to Revolutionize Chronic Lymphocytic Leukemia Research
Recently, the TriNetX Oncology team and their partners harnessed the power of RWD in a retrospective observational study to investigate the factors influencing first-line (1L) treatment decisions for CLL in a real-world setting in Germany. The study was prompted by the approval of new drugs, including ibrutinib, acalabrutinib and zanubrutinib, which target Bruton’s Tyrosine Kinase (BTK), and venetoclax, which targets B-cell lymphoma 2 (BCL2). In the context of CLL, BTK and BCL2 are key proteins involved in the disease’s pathogenesis and treatment.
Prior to the TriNetX research study, limited observational data was available on the implementation of these new drugs for the treatment of CLL in actual clinical practice. We spoke with Zuzana Dostalova, Data Scientist at TriNetX Oncology, about the research study.

Empowering Better Clinical Trial Designs with TriNetX LIVE™
Download a PDF overview of TriNetX LIVE™: A no-code, all-powerful interface for clinical research.
TriNetX LIVE™ is the world’s largest federated network of real-world data, harmonizing EHR, laboratory results, tumor registry information, and more into a common data model for on-demand cohort creation and analysis. The TriNetX LIVE™ platform aggregates recent and longitudinal observations on more than 300 million de-identified patients who have received or are receiving
care across the globe.
TriNetX Publications for European Oncology Research
Delve into our archive of past publications to gain insights into the latest trends and advancements in oncology research in Europe. Each publication underscores our commitment to advancing the field and contributing valuable knowledge to the global healthcare community. From cutting-edge methodologies to real-world data analyses, publications using TriNetX are widely recognized for their quality and relevance.
Let’s Talk
Reserve your time with TriNetX experts live at ESMO 2024. Space is limited.
Our Mission is Our Promise
At TriNetX, our mission is to operate the world’s broadest federated network of real-world data in partnership with healthcare providers and apply intelligence that accelerates innovation across the healthcare ecosystem.
Access and Analyze Rich, Secure, Real-Time Data
TriNetX is a global network of healthcare organizations and life science companies, driving real-world research to accelerate the development of life-saving therapies.
From trial operations to evidence generation, we put you at the forefront of discovery.
Clinical Trial Design & Optimization
Datasets & Analytics
Non-US Oncology
Real-World Evidence Generation
Pharmacovigilance & Drug Safety
Book a Meeting at ESMO
Please complete the below to request a meeting at ESMO 2024 in Barcelona, Spain September 13-17, 2024.



