Case Studies & Publications
Transforming Clinical Research. Advancing Healthcare.
TriNetX is dedicated to bridging the gap between clinical research and patient care through our cutting-edge data platform. Our case studies and publications reflect our commitment to providing actionable insights, fostering collaboration, and driving innovation in healthcare research.
Want to learn more about how TriNetX can support your research initiatives? Contact us for more information or schedule a demo to see our platform in action.
See TriNetX Case Studies
See TriNetX Scientific Publications
See TriNetX Publication Guidelines
See TriNetX Data Privacy Attestation
TriNetX Case Studies
Dive into our collection of case studies highlighting how TriNetX is making a tangible difference in healthcare. Our case studies offer a detailed look at how organizations are leveraging our platform to drive impactful research, streamline clinical trials, and accelerate the journey from data to discovery.
See TriNetX Client Testimonials
FEATURED CASE STUDY
Sanofi UK and Royal Berkshire NHS Foundation Trust Optimize Clinical Trial Recruitment with Technology-Driven Feasibility and Site Selection Process
FEATURED CASE STUDY
Breaking Barriers in Lupus Research: UT Southwestern Advances Recruitment Through Real-World Data and Predictive Analytics
CASE STUDY

CRO Achieves Rapid Response, Increased Acceptance Rate Via Data-Driven Clinical Trial Site ID and Outreach Approach
CASE STUDY

East Lancashire Hospitals NHS Trust Leverages TriNetX to Automate Research Capabilities and Optimize Clinical Trial Patient Recruitment
CASE STUDY

Rapid Response and Deep Dive: University of New Mexico’s Clinical & Translational Science Center Simplifies Analytics and Accelerates Time to Insight with TriNetX Platform
CASE STUDY
Medical College of Wisconsin Leverages the TriNetX Platform to Win Grant Funding to Study Outcomes and Risk Factors for Sickle Cell Disease Patients with COVID-19
CASE STUDY

TriNetX Helps Cuyahoga County’s MetroHealth System in Ohio Strive for Clinical Research Leadership Through Data Sharing
CASE STUDY

University of Oxford, U.K. Uses TriNetX Platform to Conduct Research on Mental Health Impact of COVID-19
CASE STUDY

Penn State Increases Access to Data, Enhances Research Efforts by Joining TriNetX Research Network
CASE STUDY

Expanding and Accelerating Research at the University of Texas Medical Branch (UTMB) Blocker Burn Unit with TriNetX
CASE STUDY

Enhancing Research Studies, Increasing Clinical Trial Opportunities at West Virginia Clinical and Translational Science Institute
Recent TriNetX Scientific Publications
Stay informed with the latest research and findings published by our partners and team members. Our publications section features peer-reviewed articles, white papers, and reports that demonstrate the impact of TriNetX’s technology in the field of clinical research.
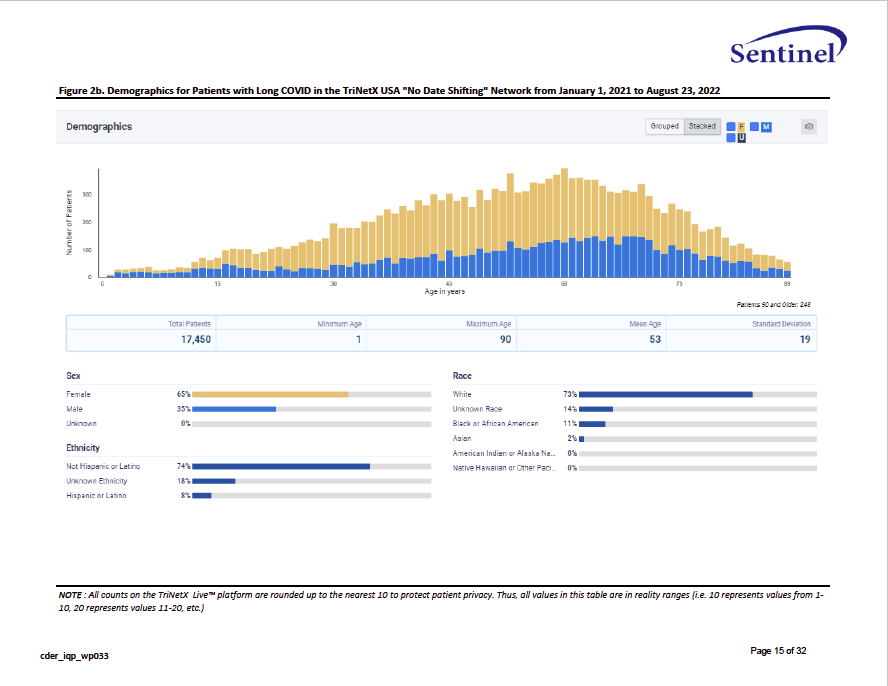
Capture of Long COVID in TriNetX: A Descriptive Analysis
Sentinel Modular Program Report. FDA Sentinal Initiative. (2024). https://www.sentinelinitiative.org/studies/drugs/individual-drug-analyses/capture-long-covid-trinetx-descriptive-analysis
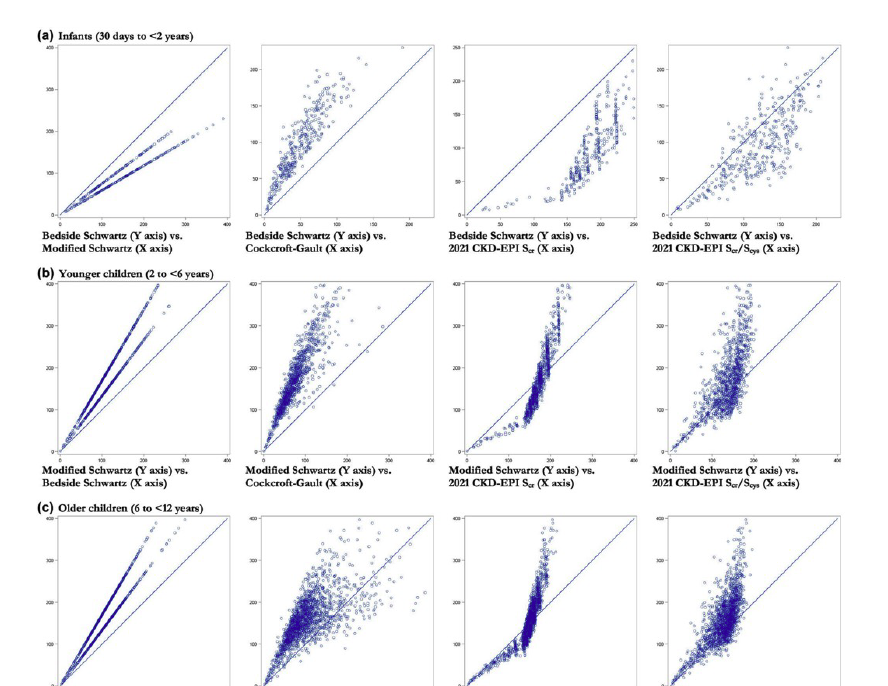
Comparing five equations to estimate glomerular filtration rate or creatinine clearance and assign individuals to KDIGO categories across the full age spectrum using real world data
, , , et al. Clin Transl Sci. 2024; 17:e13726. DOI: 10.1111/cts.13726
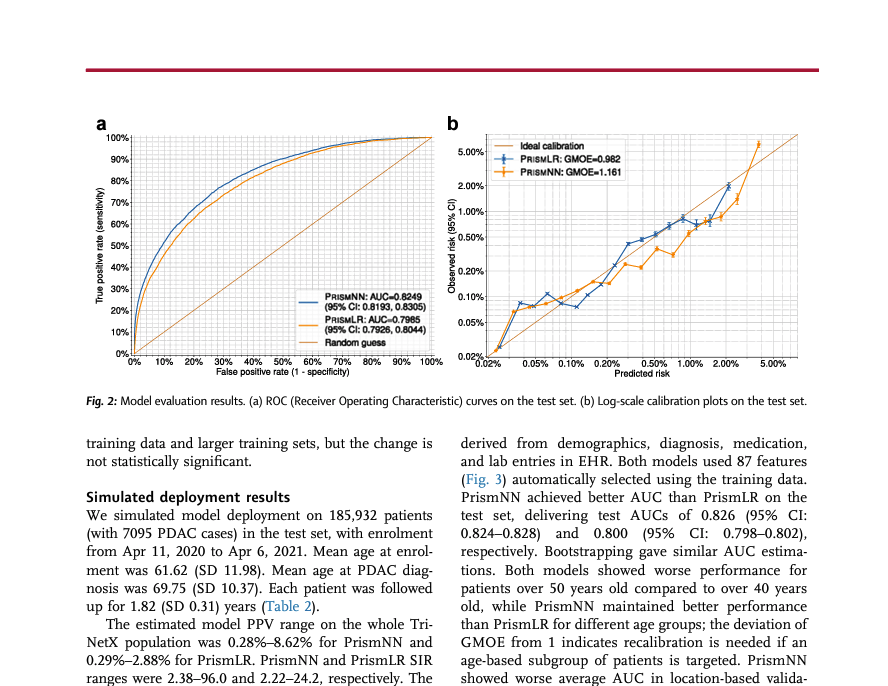
A pancreatic cancer risk prediction model (Prism) developed and validated on large-scale US clinical data
Jia K, Kundrot S, Palchuk MB, et al. eBioMedicine, vol. 98, Dec. 2023, p.104888. DOI: 10.1016/j.ebiom.2023.104888
TriNetX Colorectal Research Publications
Did the uptake of new treatment options change the treatment strategy in patients with colorectal cancer and primary non-resectable metastases?
The results of large population-based survey in Germany 2005-2007
TriNetX Esophagogastric Research Publications
Trastuzumab in Esophagogastric Cancer
HER2-Testing and Treatment Reality outside Clinical Studies in Germany
TriNetX Gastric Research Publications
TriNetX Lung Research Publications
TriNetX Lymphoma/Leukemia Research Publications
Behandlung indolenter Lymphome in Deutschland
Ergebnisse einer repräsentativen nationalen Erhebung
Indolente Non Hodgkin Lymphome
Trends in der Diagnose und in der Therapie in Deutschland (2006-2009)
TriNetX MDS Research Publications
TriNetX Multiple Myeloma Research Publications
Treatment Situation for Newly Diagnosed Multiple Myeloma Patients in Germany
First Results from a Representative Multicentre Treatment Survey
Role of Bortezomib in the Treatment of Multiple Myeloma
First Results from a Representative Multicentre Treatment Survey in Germany
Anwendung neuer Therapien zur Behandlung des multiplen Myeloms
Ergebnisse einer repräsentativen multizentrischen Erhebung 2006
Treatment of multiple myeloma in Germany
An update of a representative multicentre health care survey 2004‐2011
Diagnostic and therapeutic approaches to multiple myeloma patients
‘Real-world’ data from representative multicentre treatment surveys in Germany between 2008 and 2011
Diagnosis and treatment of multiple myeloma in Germany
Analysis of a nationwide, multi-institutional survey
Evolving Treatment Trends in Relapsed/Refractory Multiple Myeloma in Europe from 2016 to 2018
Analysis of a Multi-National Survey
Comparative Effectiveness of Pomalidomide Plus Low-Dose Dexamethasone (POM+LoDEX) in Relapsed and Refractory Multiple Myeloma
Use of Real-World Data in the Absence of Head-to-Head Studies
TriNetX Oncology GmbH is a wholly owned subsidiary of TriNetX, LLC.
TriNetX Contact Information for Physicians in Europe:
Tel: +49 761 – 38 39 94 – 0 | Fax: +49 761 – 20 25 117 | oncology@trinetx.com
Featured Pharmacovigilance Resources

Use of Electronic Health Record Data for Drug Safety Signal Identification: A Scoping Review
To evaluate the current state of EHR-based medication safety signal identification, we conducted a scoping literature
review of studies aimed at identifying safety signals from routinely collected patient-level EHR data. We extracted
information on study design, EHR data elements utilized, analytic methods employed, drugs and outcomes evaluated, and
key statistical and data analysis choices.

Using Real-World Data for Rapid Safety Investigations: Value of Near-Real-Time Clinical Data Querying
Our main objective is to investigate whether an EHR-based data network can be used in pharmacovigilance investigations to calculate background rates, using rates of anemia in non-small cell lung cancer (NSCLC) patients and an illustrative use case.
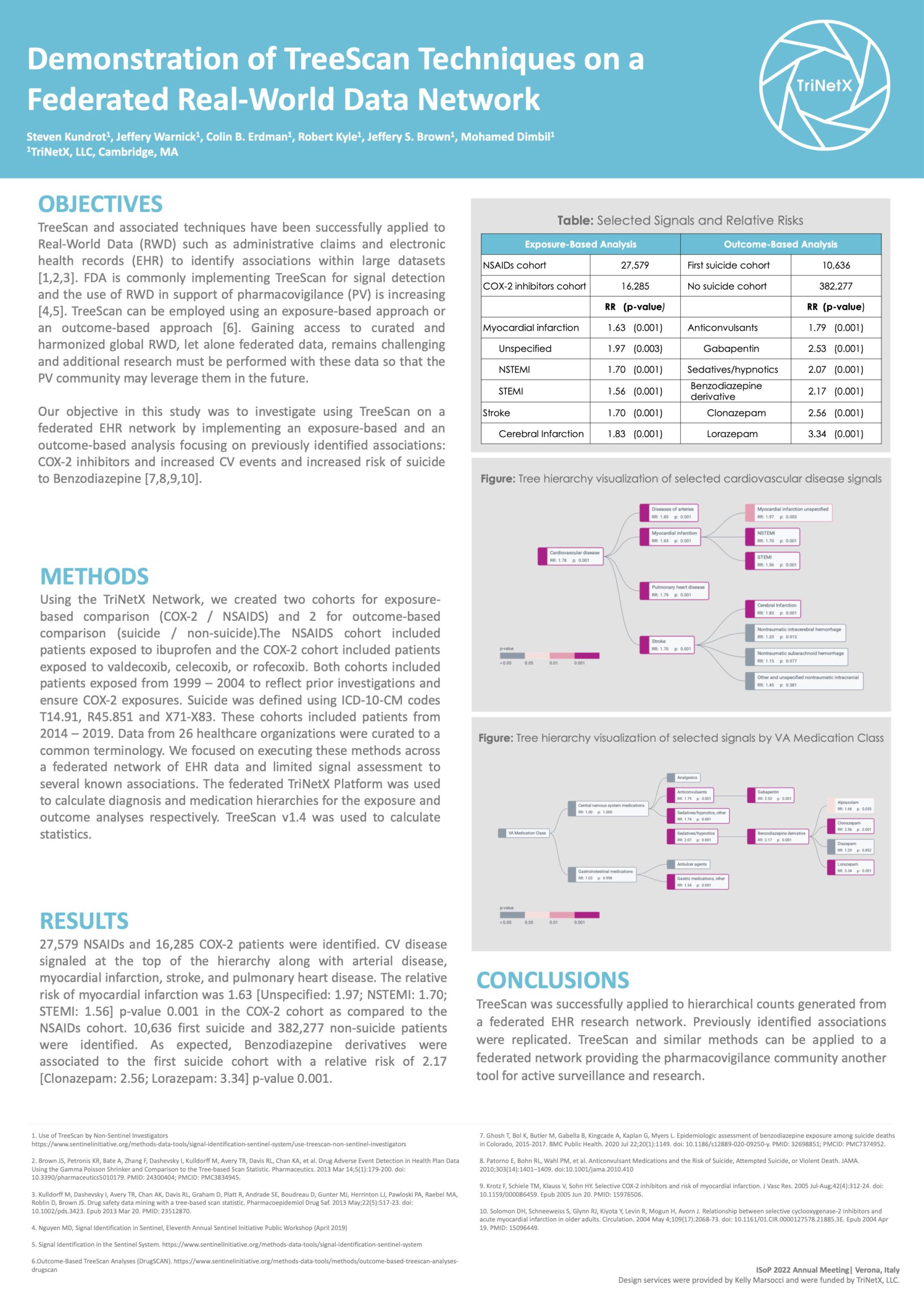
Demonstration of TreeScan Techniques on a Federated Real-World Data Network
Our objective in this study was to investigate using TreeScan on a federated EHR network by implementing an exposure-based and an outcome-based analysis focusing on previously identified associations.

The Complete Guide to GVP Module IX for Signal Management
This guide is intended to provide a comprehensive overview of GVP-IX and discuss how EVIDEX® Signal Management, a next generation pharmacovigilance workflow platform can help organizations detect, track, and resolve safety signal and inquiries in compliance with GVP-IX.
Published Pharmacovigilance Research

A Pharmacovigilance Signaling System Based on FDA Regulatory Action and Post-Marketing Adverse Event Reports
Many serious drug adverse events (AEs) only manifest well after regulatory approval. Therefore, the development of signaling methods to use with post-approval AE databases appears vital to comprehensively assess real-world drug safety. However, with millions of potential drug–AE pairs to analyze, the issue of focus is daunting.

Development of a drug safety ePlatform for physicians, pharmacists, and consumers based on post-marketing adverse events
Hoffman, K. B., Overstreet, B. M., & Doraiswamy, P. M. (2013). Drugs and Therapy Studies, 3(1), e4-e4.

The Weber Effect and FAERS: Analysis of Sixty-Two Drugs Approved from 2006 to 2010
Most of the modern adverse event reporting into FAERS does not follow the pattern described by the ‘Weber Effect’. Modern FAERS reporting has improved greatly over the last decade and will be an increasingly important factor in determining the overall safety profile of FDA approved prescription drugs

Stimulated Reporting: The Impact of US Food and Drug Administration-Issued Alerts on the Adverse Event Reporting System (FAERS)
“Stimulated reporting” is the concept that public disclosure of a safety issue by the issuance of an FDA alert or warning, or the clustering of adverse event reports triggered by consumer-based support group and/or litigation, will result in substantially increased reporting rates for the affected drug. This publication refutes this widely held assumption.

Neuropsychiatric adverse effects of oseltamivir in the FDA Adverse Event Report System, 1999-2012
Publications from Roche and a case-control study suggest that there is no evidence, or plausible mechanism of action, to link neuropsychiatric adverse events to Tamiflu (oseltamivir). Cochrane Collaborators, the BMJ, and others, however, contend that many of Roche’s data remain unavailable.

A Survey of the FDA's AERS Database Regarding Muscle and Tendon Adverse Events Linked to the Statin Drug Class
Cholesterol management drugs known as statins are widely used and usually well tolerated. However, a variety of muscle-related side effects can arise. We conducted a review of post-approval muscle and tendon AE reports in association with statin use, to assess differences within the drug class.

Analysis of Spontaneous Postmarket Case Reports Submitted to the FDA Regarding Thromboembolic Adverse Events and JAK Inhibitors
A systematic review of FAERS found elevated reporting for both tofacitinib and ruxolitinib for certain thromboembolic adverse events.

Serious Cardiovascular Adverse Events Reported with Intravenous Sedatives: A Retrospective Analysis of the MedWatch Adverse Event Reporting System.
Duprey, M. S., Al-Qadheeb, N. S., O’Donnell, N., Hoffman, K. B., Weinstock, J., Madias, C., … & Devlin, J. W. (2019). Drugs-Real World Outcomes, 6(3), 141.

A Drug Safety Rating System Based on Post-Marketing Costs Associated with Adverse Events and Patient Outcomes
Hoffman, K. B., Dimbil, M., Kyle, R. F., Tatonetti, N. P., Erdman, C. B., Demakas, A., … & Overstreet, B. M. (2015). Journal of managed care & specialty pharmacy, 21(12), 1134-1143c.

Post-approval adverse events of new and old anticoagulants
Hoffman, K. B., Demakas, A., Erdman, C. B., & Dimbil, M. (2014). BMJ (Clinical research ed.), 348, g1859.
Pharmacovigilance Poster Presentations

PAINWeek 2020: Adverse Events Associated with Analgesics Used for Osteoarthritis Pain: Analysis of Post-Marketing Data
Bannuru, R.R., Dimbil, M., Wei, W., Colilla, S., Huyghues-Despointes, C., Nettleship, J.E., Iyer, R. (2020, September). Adverse Events Associated with Analgesics Used for Osteoarthritis Pain: Analysis of Post-Marketing Data. Poster presented at PAINWeek 2020 National Conference on Pain Management.
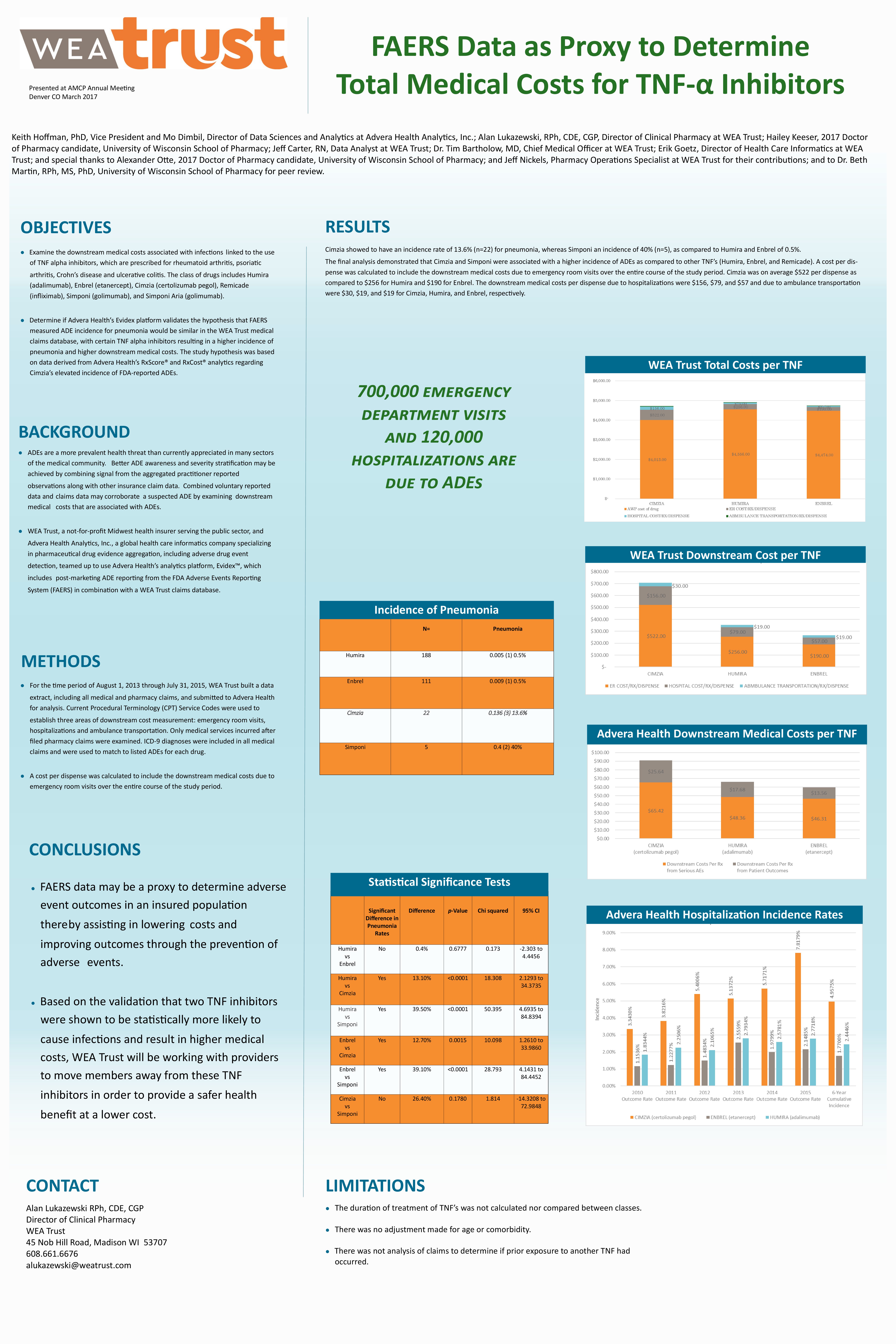
AMCP 2017: FAERS Data as Proxy to Determine Total Medical Costs for TNF-α Inhibitors
Hoffman, K., Dimbil, M., Lukazewski, A., Keeser, H., Carter, J., Bartholow, T., & Goetz, E. (2017, March). FAERS Data as Proxy to Determine Total Medical Costs for TNF-α Inhibitors. Poster presented at the Academy of Managed Care Pharmacy (AMCP) Meeting, Denver, CO.
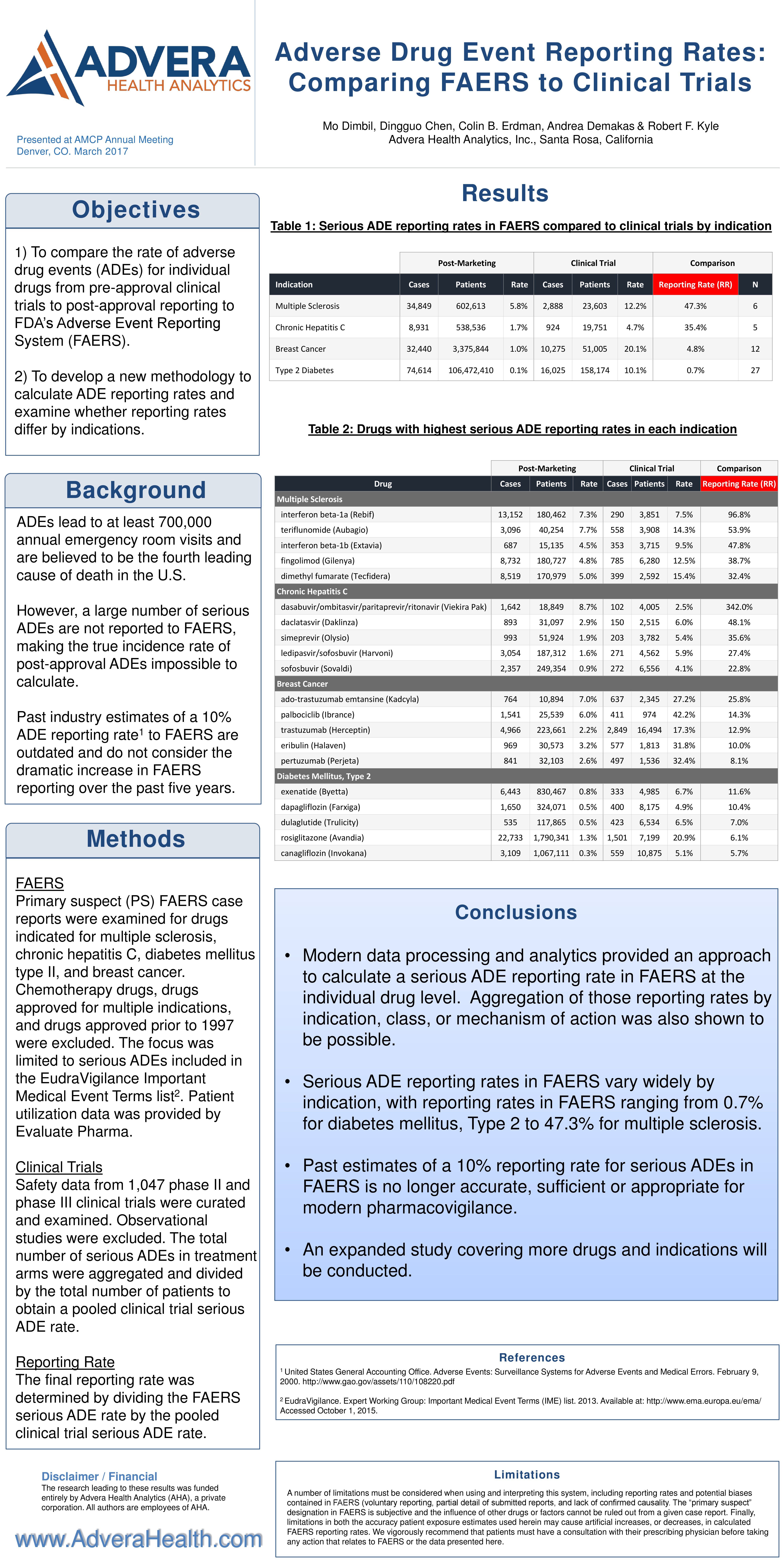
AMCP 2017: Adverse Drug Event Reporting Rates: Comparing FAERS to Clinical Trials
Compares the rate of adverse drug events (ADEs) for individual drugs from pre-approval clinical trials to post-approval reporting to FDA’s Adverse Event Reporting System (FAERS).

EHDN 2016: Post-Marketing Adverse Events Associated with Tetrabenazine: Findings Using the FDA Adverse Event Reporting System
Classen, D.O., Iyer, R., Dimbil, M., Giron, A., De Boer, L.M., Gandhi, S., & Hoffman, K.B. (2016, September). Post-Marketing Adverse Events Associated with Tetrabenazine: Findings Using the FDA Adverse Event Reporting System. Poster presented at the 9th European Huntington’s Disease Network (EHDN) Plenary Meeting, The Hague, The Netherlands.
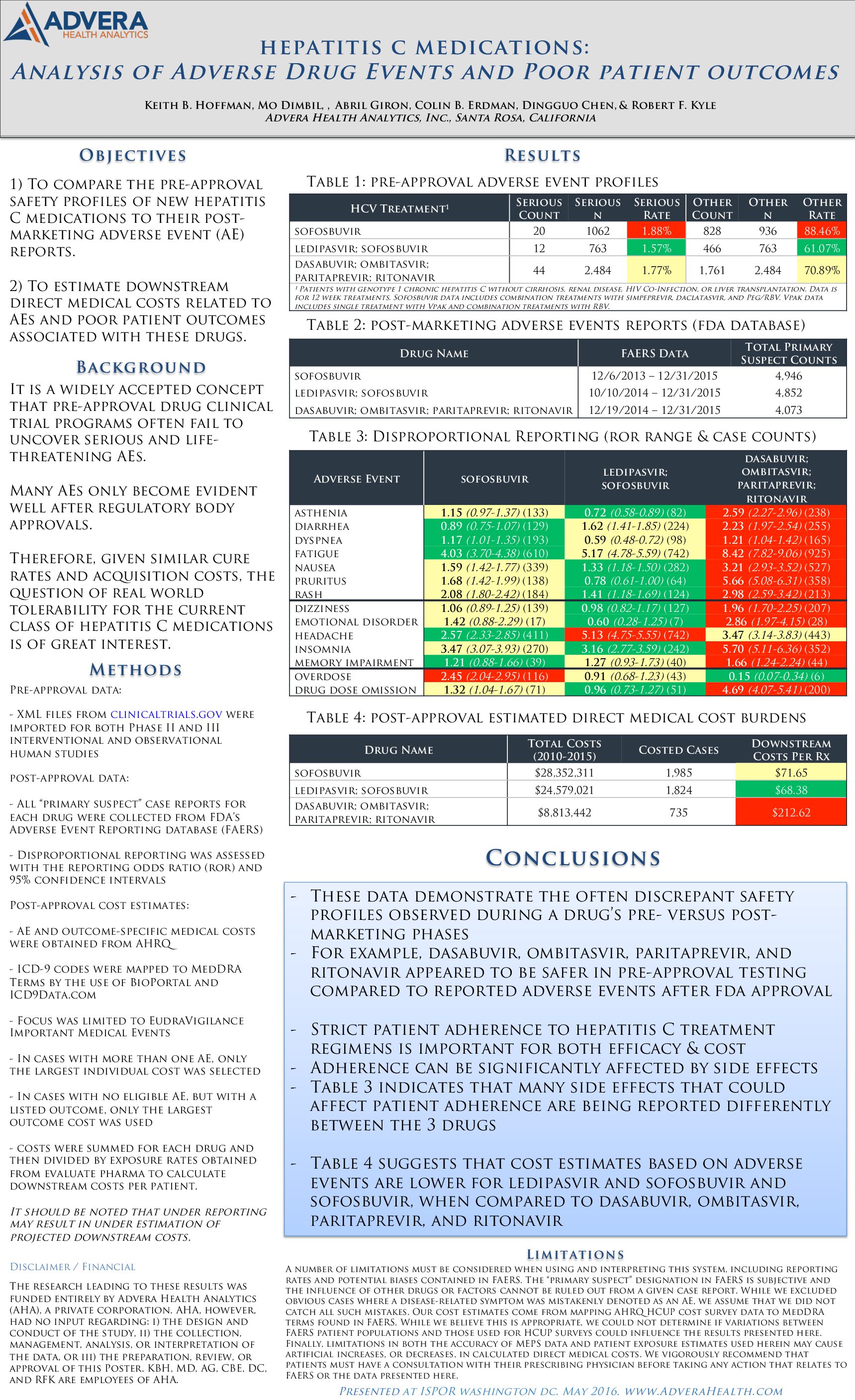
ISPOR 2016: Hepatitis C Medications: Analysis of Adverse Drug Events and Poor Patient Outcomes
Hoffman, K.B., Dimbil, M., Giron, A., Erdman, C.B., Chen, D., & Kyle, R.F. (2016, May). Hepatitis C Medications: Analysis of Adverse Drug Events and Poor Patient Outcomes. Poster presented at ISPOR, Washington, DC.
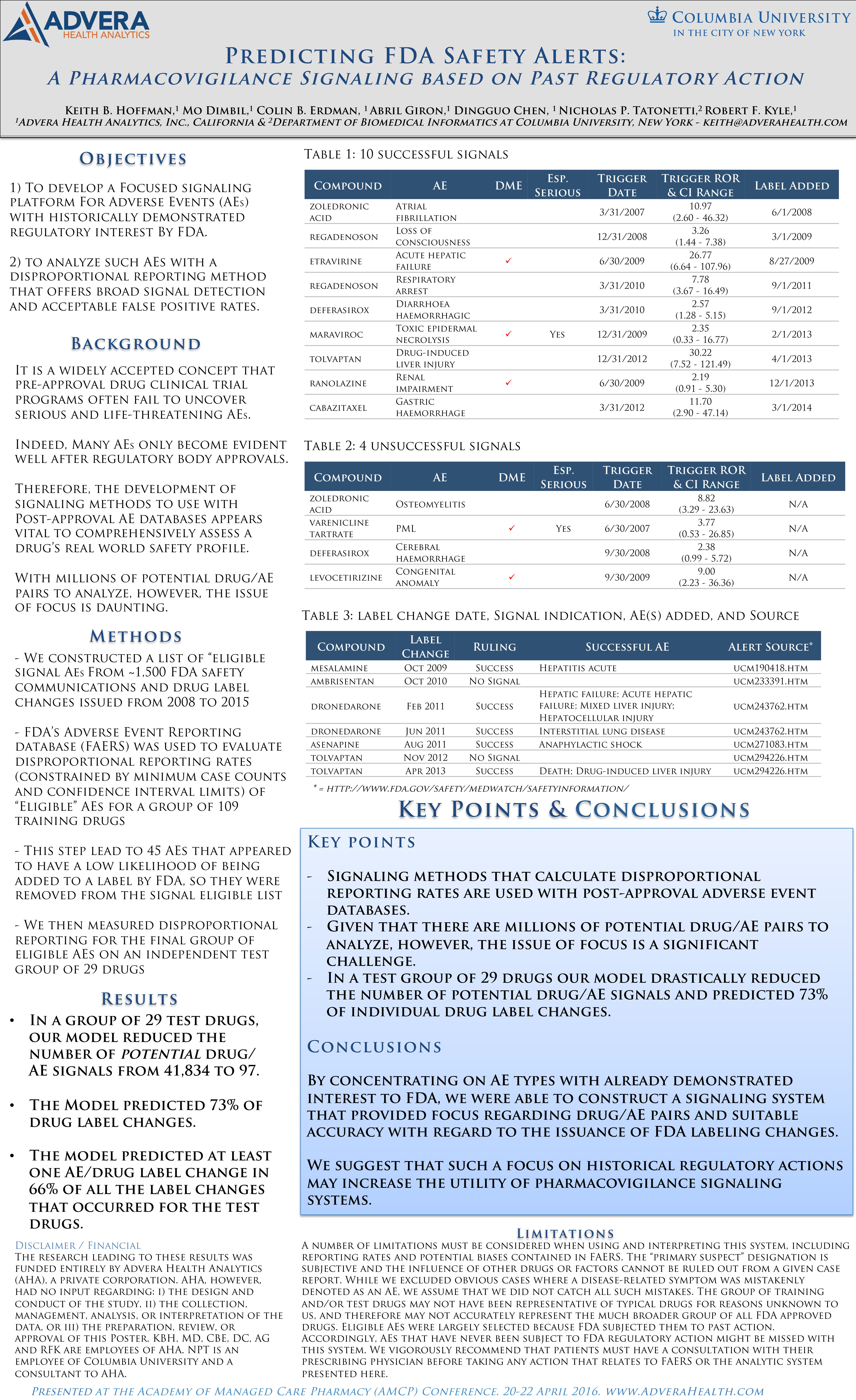
AMCP April 2016: Predicting FDA Safety Alerts: A Pharmacovigilance Signaling System Based on Past Regulatory Action
Hoffman, K.B., Dimbil, M., Erdman, C.B., Giron, A., Chen, D., Tatonetti, N.P., & Kyle, R.F. (2016, April). Predicting FDA Safety Alerts: A Pharmacovigilance Signaling based on Past Regulatory Action. Poster presented at the Academy of Managed Care Pharmacy (AMCP) Meeting, San Francisco, CA.

ASHP Midyear 2015: Direct Medical Costs from Post-Marketing Adverse Drug Reactions: Focus on GLP-1, DPP-4 & SGLT2 Type 2 Diabetes Medications
Hoffman, K.B., Giron, A., & Dimbil, M. (2015, December). Direct Medical Costs from Post-Marketing Adverse Drug Reactions: Focus on GLP-1, DPP-4, & SGLT2 Type 2 Diabetes Medications. Poster presented at ASHP Midyear Clinal Meeting and Exhibition, New Orleans, LA.
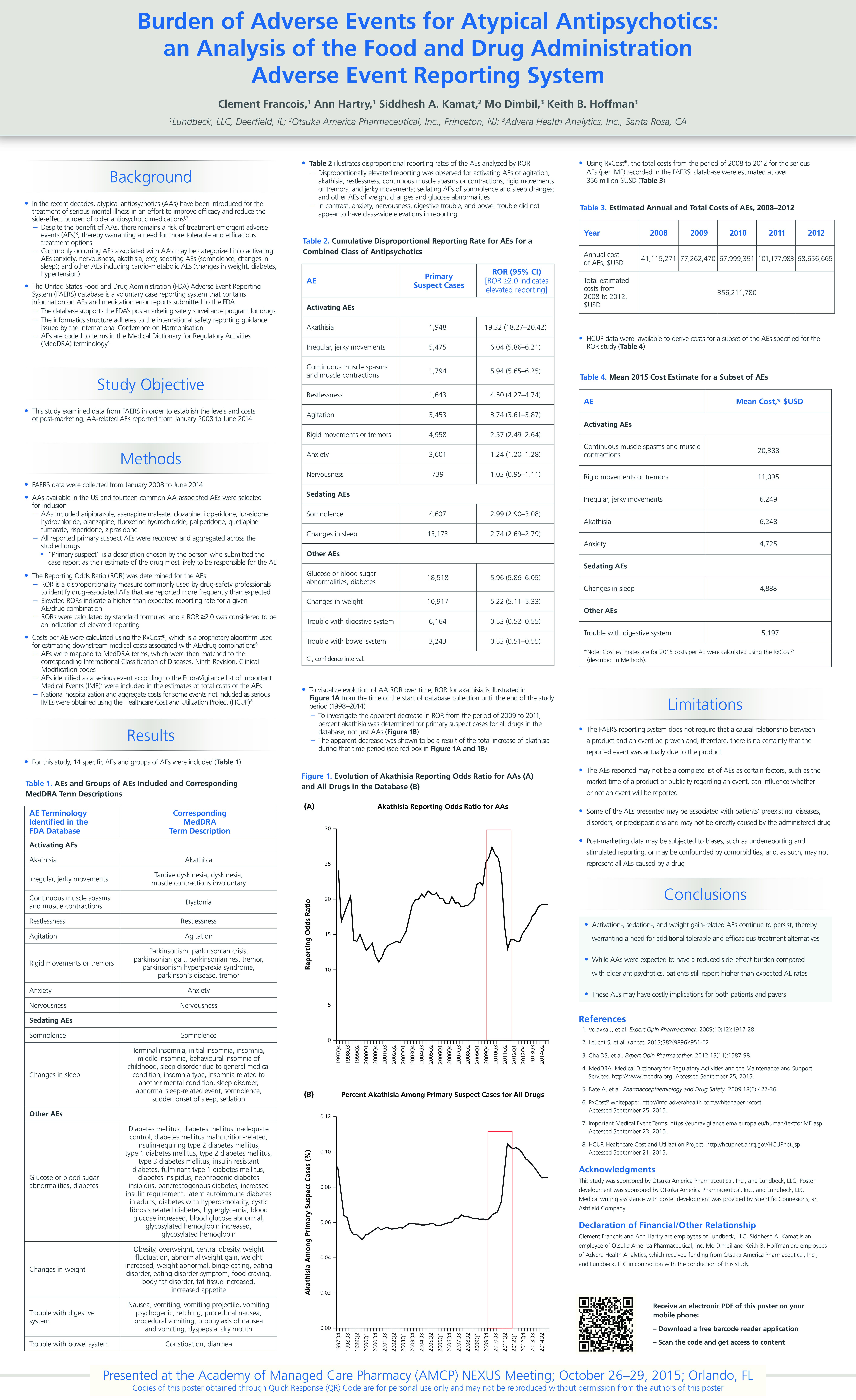
AMCP Nexus October 2015: Burden of Adverse Events for Atypical Antipsychotics: an Analysis of the Food and Drug Administration Adverse Event Reporting System
Francois, C., Harty A., Kamat, S.A., Dimbil, M., & Hoffman, K.B. (2015, October). Burden of Adverse Events for Atypical Antipsychotics: An Analysis of the Food and Drug Administration Adverse Event Reporting System. Poster presented at the Academy of Managed Care Pharmacy (AMCP) Nexus Meeting, Orlando, FL.
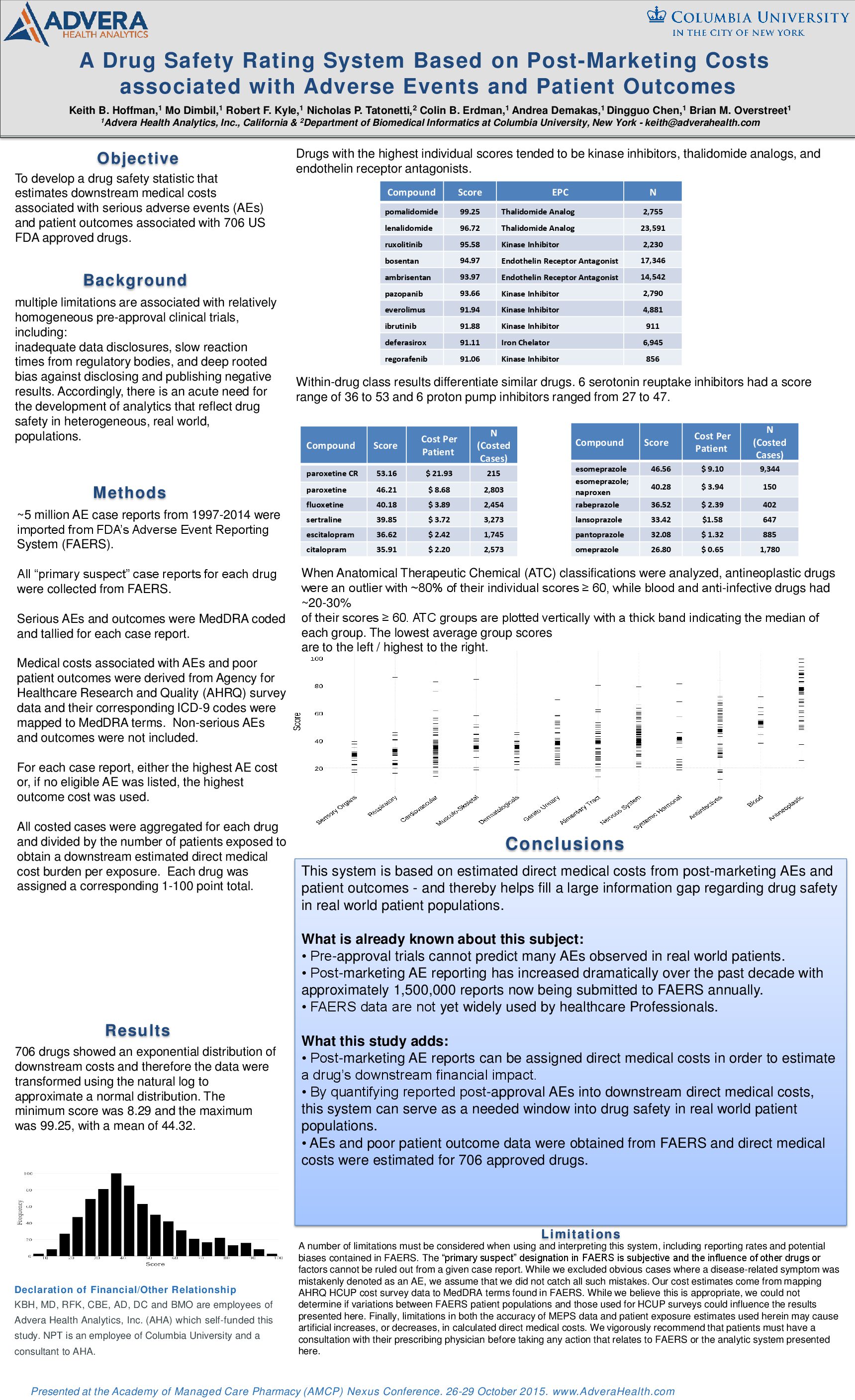
AMCP Nexus October 2015: A Drug Safety Rating System Based on Post-Marketing Costs associated with Adverse Events and Patient Outcomes
Hoffman, K.B., Dimbil, M., Kyle, R.F., Tatonetti, N.P., Erdman, C.B., Demakas, A., Chen, D., & Overstreet, B.M. (2015, October). A Drug Safety Rating System Based on Post-Marketing Costs associated with Adverse Events and Patient Outcomes. Poster presented at the Academy of Managed Care Pharmacy (AMCP) Nexus Meeting, Orlando, FL.
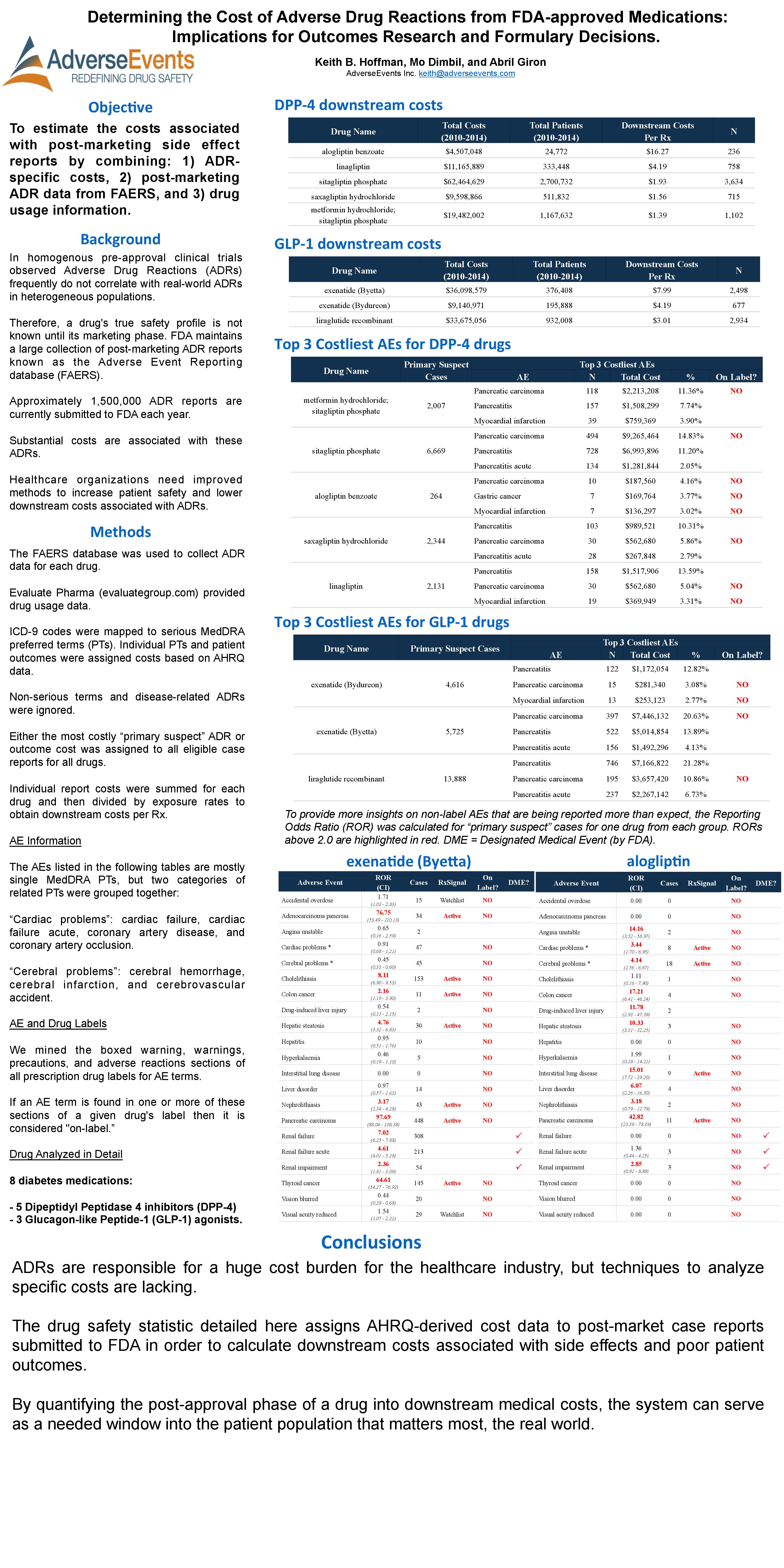
AMCP April 2015: Determining the Cost of Adverse Drug Reactions from FDA-approved Medications: Implications for Outcomes Research and Formulary Decisions
Hoffman, K.B., Dimbil, M., & Giron, A. (2015, April). Determining the Cost of Adverse Drug Reactions from FDA-approved Medications: Implications for Outcomes Research and Formulary Decisions. Poster presented at the Academy of Managed Care Pharmacy (AMCP) Meeting, San Diego, CA.
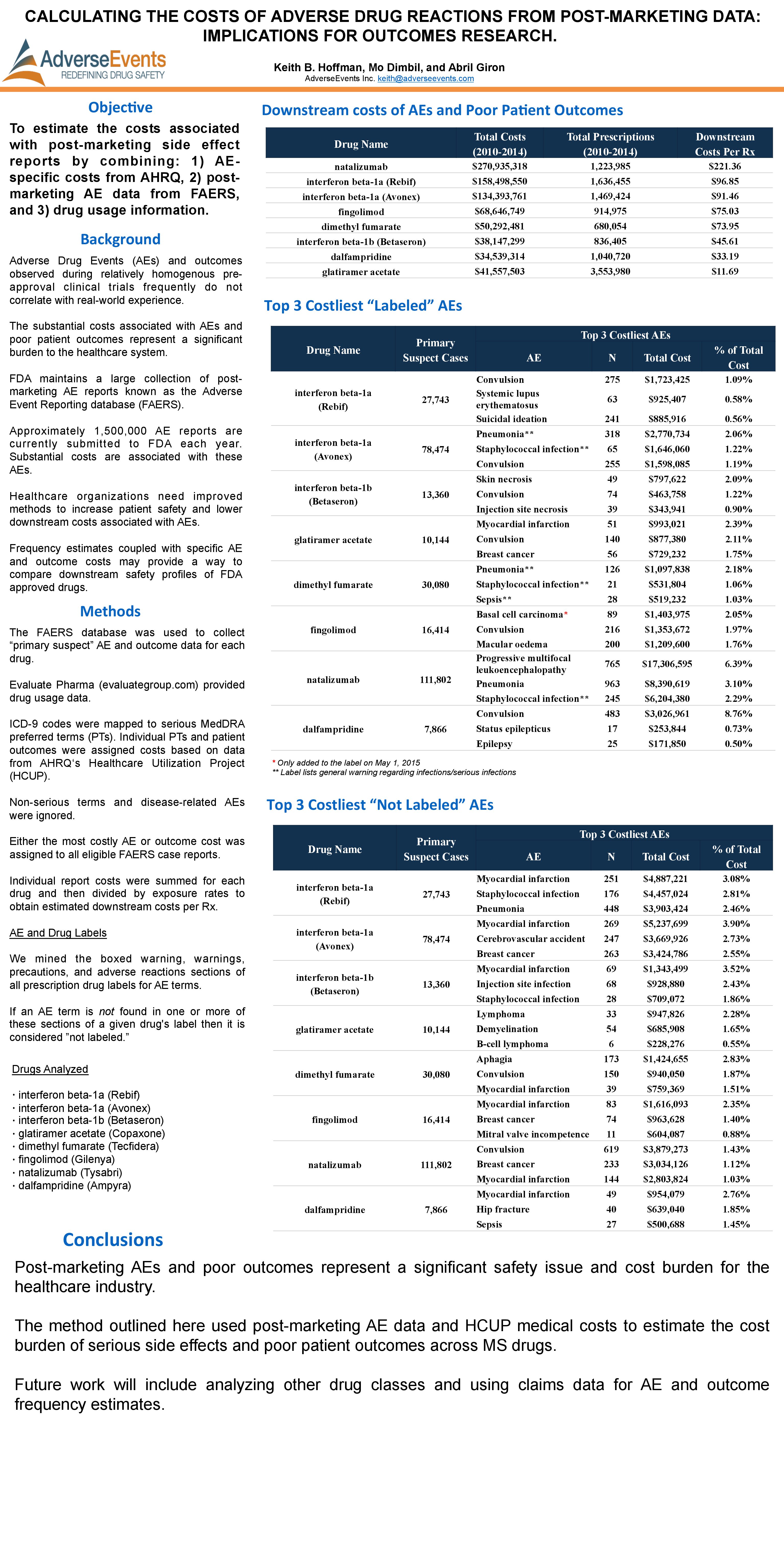
ISPOR 2015: MS Drugs: Calculating the Costs of Adverse Drug Reactions from Post-Marketing Data: Implications for Outcomes Research
Hoffman, K.B., Dimbil, M., & Giron, A. (2015, May). MS Drugs: Calculating the Costs of Adverse Drug Reactions from Post-Marketing Data: Implications for Outcomes Research. Poster presented at ISPOR, Philadelphia, PA.
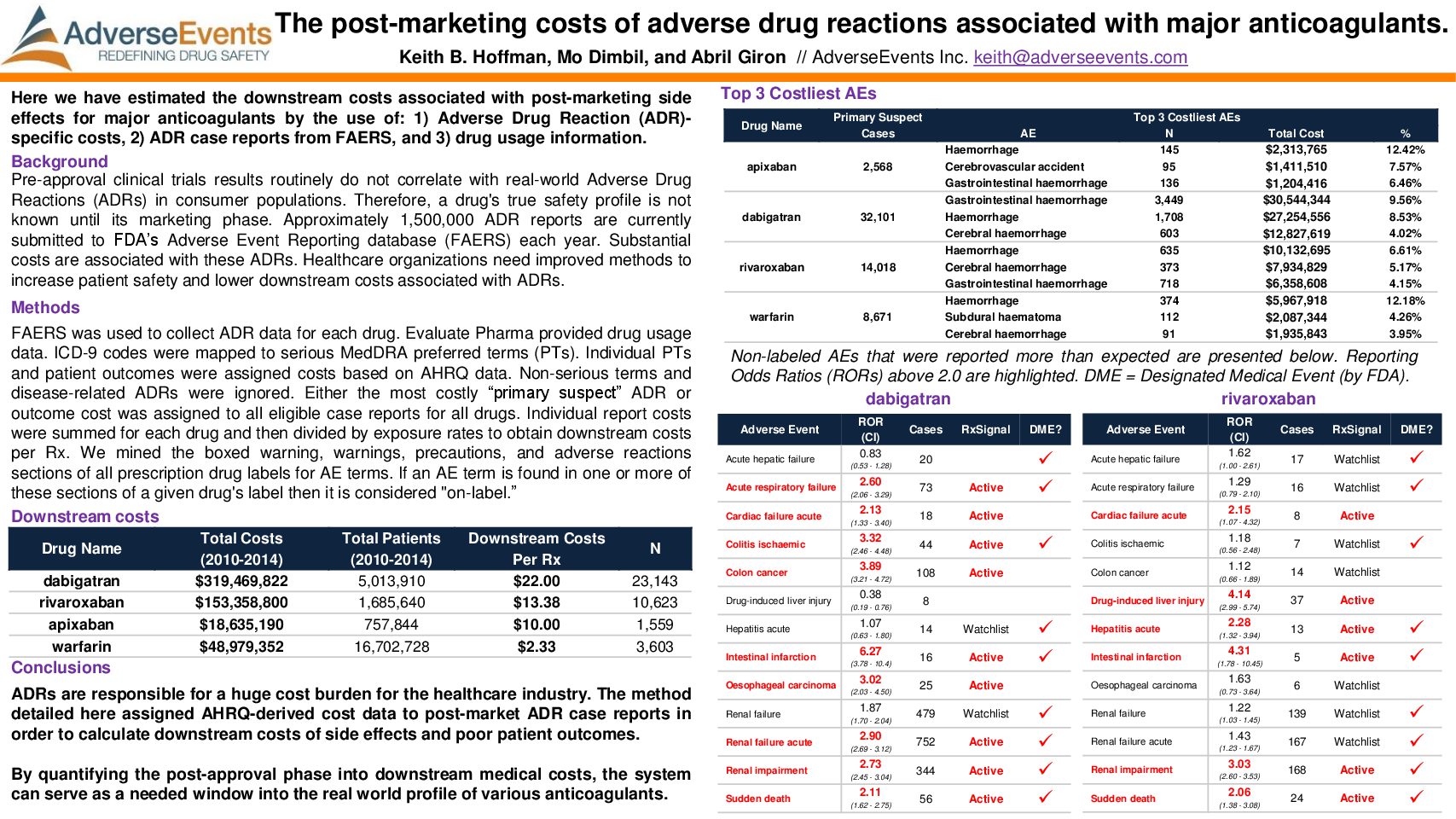
Anticoagulation Forum: The Post-Marketing Costs of Adverse Drug Reactions Associated with Major Anticoagulants
Hoffman, K.B., Dimbil, M., & Giron, A. The Post-Marketing Costs of Adverse Drug Reactions Associated with Major Anticoagulants. Poster presented at the Anticoagulation Forum.
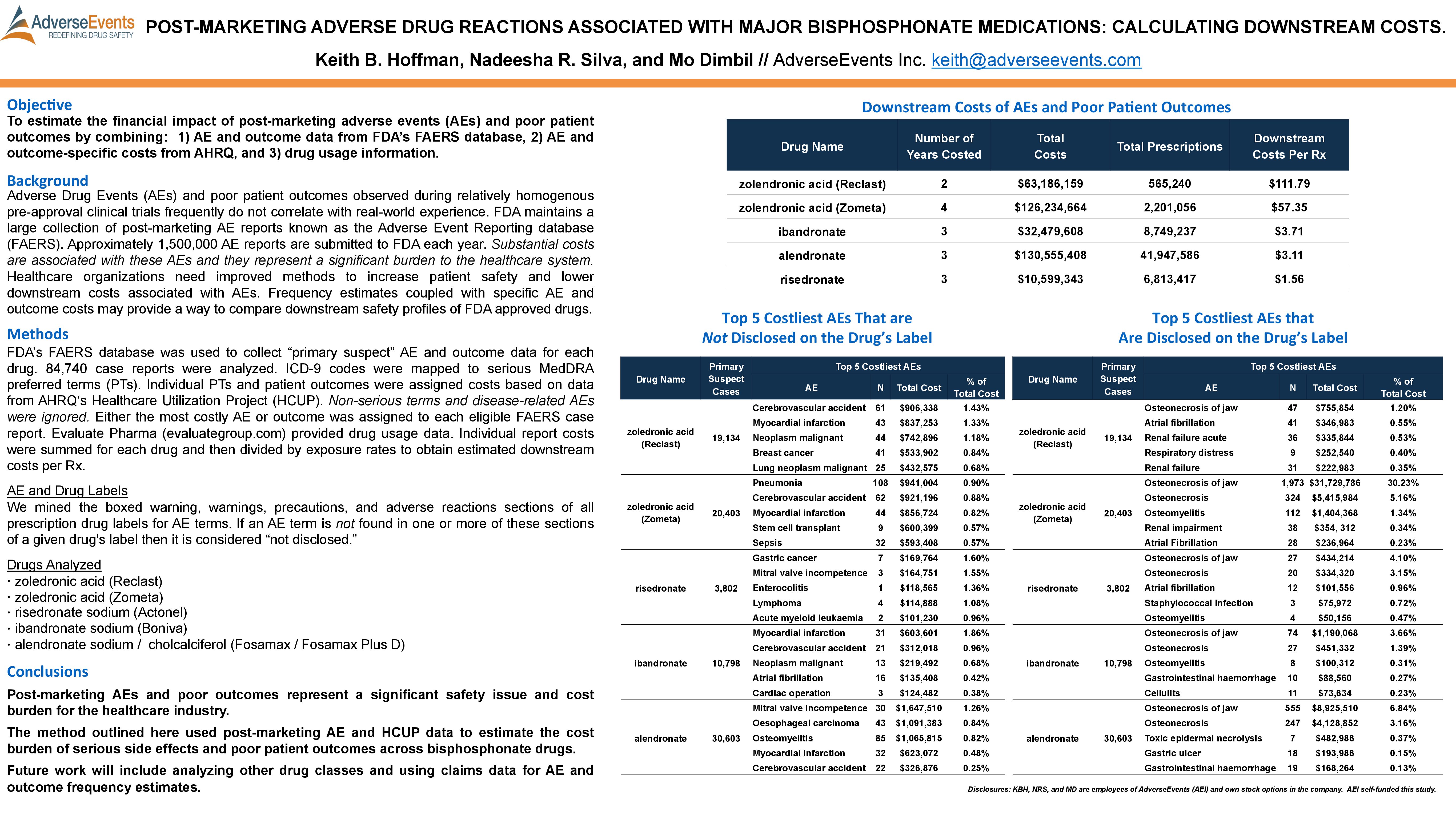
ASHP 2015: Post-Marketing Adverse Drug Reactions Associated with Major Bisphosphonate Medications: Calculating Downstream Costs
Hoffman, K.B., Silva, N.R., & Dimbil, M. (2015, June). Post-Marketing Adverse Drug Reactions Associated with Major Bisphosphonate Medications: Calculating Downstream Costs. Poster presented at ASHP Summer Meeting and Exhibition, Denver, CO.
TriNetX Drug Safety White Papers

The Complete Guide to GVP Module IX for Signal Management
This guide is intended to provide a comprehensive overview of GVP-IX and discuss how EVIDEX® Signal Management, a next generation pharmacovigilance workflow platform can help organizations detect, track, and resolve safety signal and inquiries in compliance with GVP-IX.

White Paper: Pharmacovigilance Software in 2021
Analysis of the PV software market in 2021 and beyond, including trends and comparisons between legacy vs. next generation software.

Pharmacovigilance Best Practices for Clinical and Early Post-Approval Biopharma
The idea of selecting, implementing, and deploying pharmacovigilance software is overwhelming for smaller companies. Smaller clinical and early post-approval stage biopharma companies have to make do with significantly fewer resources and they are understandably quick to look for outside help.
Analysis of Post-Marketing Safety Signals for COVID-19 Therapies
We reviewed the top 6 most widely used COVID-19 treatments using Q2 2020 FAERS data. The report includes new potential safety signals, dechallenge and rechallenge data, detailed signal analysis and ROR forest plots, and top 10 highest reported adverse event tables for each product.
EVIDEX® is a registered trademark of Advera Health Analytics, Inc., which is a wholly owned subsidiary of TriNetX, LLC.