GVP Module IX for Signal Management: The Complete Guide
Introduction
Good Pharmacovigilance Practices (GVP) are a set of measures put into practice in 2012 to facilitate the performance of pharmacovigilance in the European Union (EU). GVP is broken out into several modules that govern different aspects of pharmacovigilance processes. GVP Module IX – Signal Management (GVP IX) provides general guidance and requirements on scientific and quality aspects of signal management.
The guidelines apply to Marketing Authorization Holders (MAH) for medicines authorized by the European Medicines Agency (EMA) and EU Member States. However, in the absence of formal regulation on the process of signal management by other health authorities, such as FDA, these guidelines have become the de facto global standard.
There is no specifications on how these requirements are met, but in the age of software-as-a-service (SaaS), technology is playing an ever bigger role.
Download the complete 18-page guide.
GVP IX Signal Detection
Signal detection should include manual review of Individual Case Safety Reports (ICSRs), statistical analyses, or a combination of both. Statistical methodologies are discussed in GVP IX Addendum I – Methodological aspects of signal detection from spontaneous reports of suspected adverse reactions. In short, disproportionality analysis in combination with additional data summaries based on both statistical and clinical considerations should be used.
An in-depth review of the signal detection process in GVP Module IX for Signal Management can be found in Pharmacovigilance Signal Detection: The Complete Guide.
GVP IX Signal Validation
Signal validation is the process of confirming a new potentially causal association, or a new aspect of a known association, of a drug-event combination. When validating a signal, GVP IX recommends that the following elements should be considered:
- Previous awareness
- Strength of evidence
- Clinical relevance and context
The full module document provides numerous examples of these elements, so won’t be discussed in detail here. However, having access to robust ICSR data, both for individual cases and in aggregate is necessary to justify further analysis.
At this stage a decision will be made to determine if the signal is non-validated or that it is in fact a validated signal that is in need of prioritization and further assessment considering all available data.
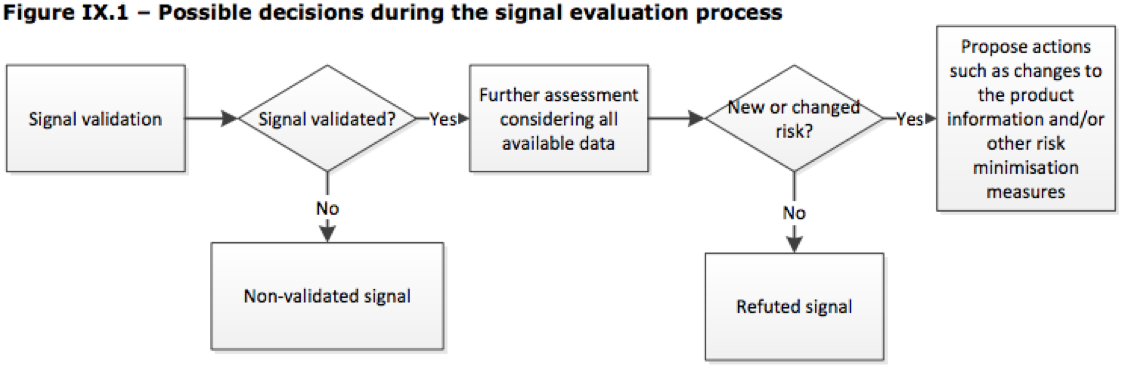
GVP IX Signal Prioritization & Assessment
After an initial review, a determination is made whether the signal is non-validated or that it is in fact a validated signal that needs prioritization and further assessment.
Once validated, a signal needs to be prioritized based on whether the signals suggest risks with “an important impact on patients’ or public health and/or the risk-benefit balance of the medicinal product”. GVP Module IX (GVP IX) suggests several considerations that should be examined. Ultimately the prioritization stage should dictate the timeframe for completing activities and actions throughout the signal management process. GVP IX specifically states that flexibility and clinical judgement be applied throughout prioritization and that “appropriate measures” around risk prevention or minimization may be required before a formal assessment of the signal is concluded.
Taking place in tandem with Signal Prioritization is the Signal Assessment process that considers all available evidence when further evaluating a validated signal. During this process the safety reviewer is looking for additional sources of information that may provide further evidence for or against a causal association, or a new aspect of a known association.
GVP IX Quality Requirements
The overarching theme throughout all stages of GVP IX, is that the processes used should be adequately documented and the steps taken in the management of a specific signal should be tracked and auditable. In short, the system needs to provide for transparency into the who, what, why, and when of the process.
The concept of software to solve the burden of tracking safety issues is not new. Legacy systems have outdated user Interfaces, long and costly implementation periods, and outdated analytic tools.
Evidex Signal Management, with a best-in-class user interface, fast validated implementation, and an advanced data-and-analytics-first approach, provides drug safety departments with a new option. The option to not settle for an intimidating user experience, long costly implementations, or a band-aide approach to data and analytics focused workflows.
You no longer need to settle for the status-quo and legacy software.
Learn more about TriNetX drug safety solutions and services.
Complete this short form and we'll connect you to one of our pharmacovigilance experts.
EVIDEX® is a registered trademark of Advera Health Analytics, Inc., which is a wholly owned subsidiary of TriNetX, LLC.
