 Signal Management
Signal Management
Comprehensive data leads to comprehensive safety.
EVIDEX® is your gateway to a next level of real-world data and longitudinal detail that goes well beyond traditional public sources. Include comprehensive, global, real-word data that empowers your team to discover, assess, validate, prioritize, and report in a thorough, transparent, and compliant manner.
- Use advanced technology to connect multiple data sources, including rich real-world data only available from TriNetX.
- Track and resolve safety signals from any data source with audit-ready and intuitive tools that fulfill regulatory duties and drive commercial success.
- Bring pools of information together seamlessly to enhance signaling algorithms and make validations and assessment more efficient.
- Accelerate more effective management, mitigation, and reporting.
Connect, discover, and refine. Advance and innovate.
Leverage an intuitive, fully integrated platform to clearly establish actionable intelligence from fresh, expansive, data sources.
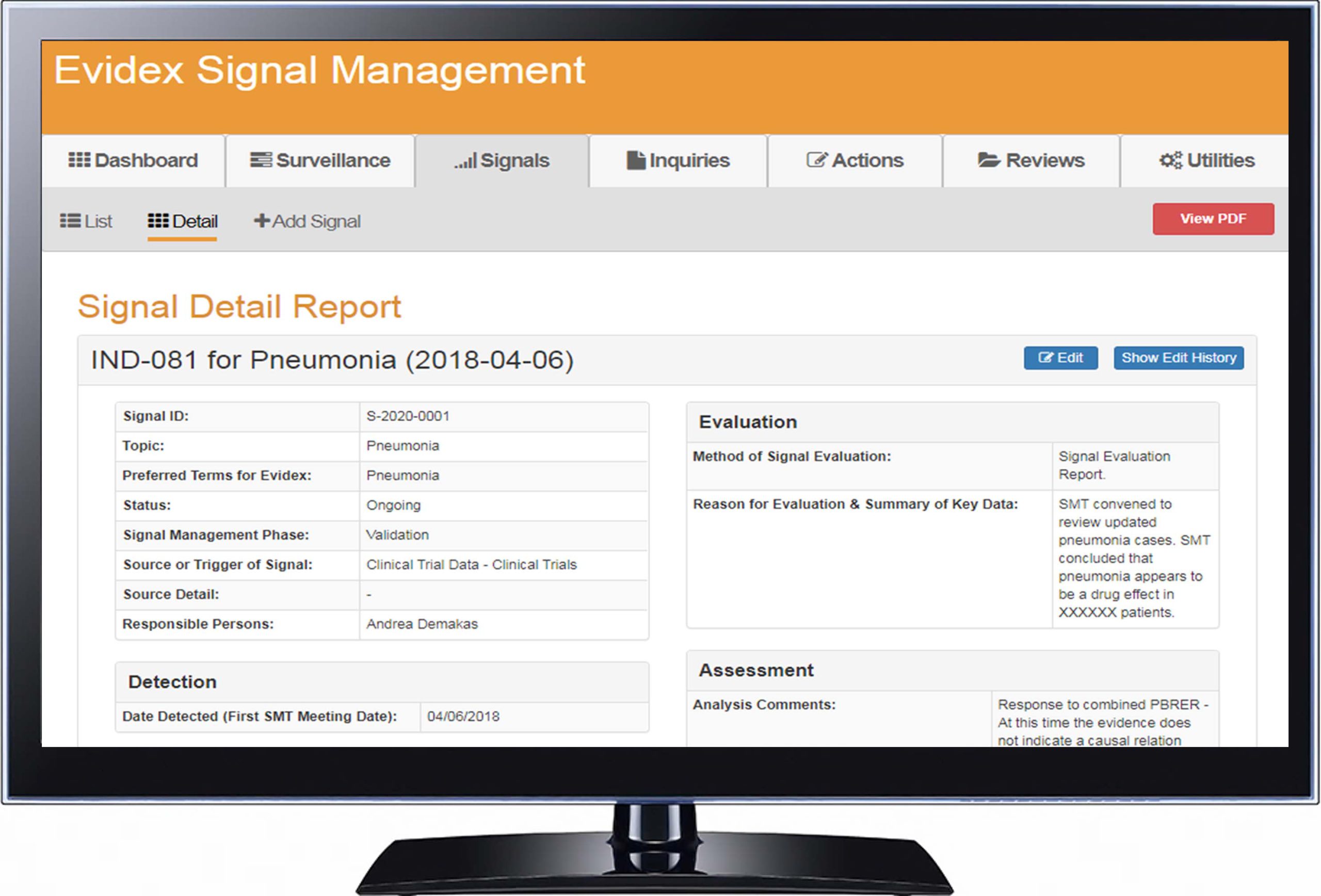
What’s Included: |
| Signals, Inquiries, Requests, and Reviews Track signals from any data source alongside internal and external inquiries and requests. |
| Easy Reporting and Audit Trail Creation Customizable filters and detailed user-level tracking ensure regulatory compliance. |
| Configurable Actions to Fit Any Workflow Actions are logged to create an audit trail. Future Actions can be created and assigned to responsible persons for an infinitely configurable workflow. |
| Fully Integrated with all Data and Analytics Seamless integration between signal detection and signal management enable tracking on any dataset. Ability to manually add signals provides for complete flexibility. |
| Off-the-Shelf GVP Module IX Compliance Good Pharmacovigilance Practices (GVP) facilitate the performance of pharmacovigilance, and is broken out into several modules. Fully validated off-the-shelf based on GVP Module IX–Signal Management (GVP IX) guidelines. |
| Best-in-Class Customer Support Learn more about our highly responsive customer service. |
Ready to take the next step?
Go beyond the limits of legacy pharmacovigilance solutions with unmatched, panoramic view of real-world, global patient data.
Request an EVIDEX® demo now:
"*" indicates required fields
EVIDEX® is a registered trademark of Advera Health Analytics, Inc., which is a wholly owned subsidiary of TriNetX, LLC.


