The use of real-world evidence (RWE) in regulatory decision-making and health technology assessments (HTA) is gaining traction, particularly in the evolving landscape of oncology pharmacotherapeutics in Europe where navigating the drug approval and market access processes is becoming more complex.
In a recent TriNetX webinar, Markus Rückert, PhD, Medical Director and Zuzana Dostalova, Data Scientist shared their perspectives on the role RWE plays in addressing regulatory complexities, the shifts in assessment approaches, and how oncology-specific challenges are being tackled using diverse data sources.
Continue reading for a recap of their presentation and for their full discussion including participant Q&A, access the webinar on-demand.
The Lifecycle of Oncology Pharmacotherapeutics and the Role of RWE
In the lifecycle of oncology drugs, RWE has the potential to elevate programs at various stages, but its impact is most crucial during the approval and market access phases. These steps are pivotal as they determine the success of new therapies in an increasingly complex regulatory environment.
Key challenges include patient subgroup availability, trial complexity, biomarker stratifications, and divergent regulatory decisions from bodies such as the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA). The demand for faster drug approval further compounds these challenges.
Traditional clinical trial approaches often fail to provide the necessary answers for these complexities, particularly during market access, where additional considerations such as cost-effectiveness and comparisons to existing standards of care are paramount.
In a continent with over 20 diverse healthcare systems, this fragmentation adds another layer of difficulty. Here, the utility of RWE becomes evident, helping bridge gaps left by clinical trials by providing insights into real-world healthcare settings, which tend to be more varied and less controlled than trial environments.
Shifts in Regulatory Acceptance of RWE
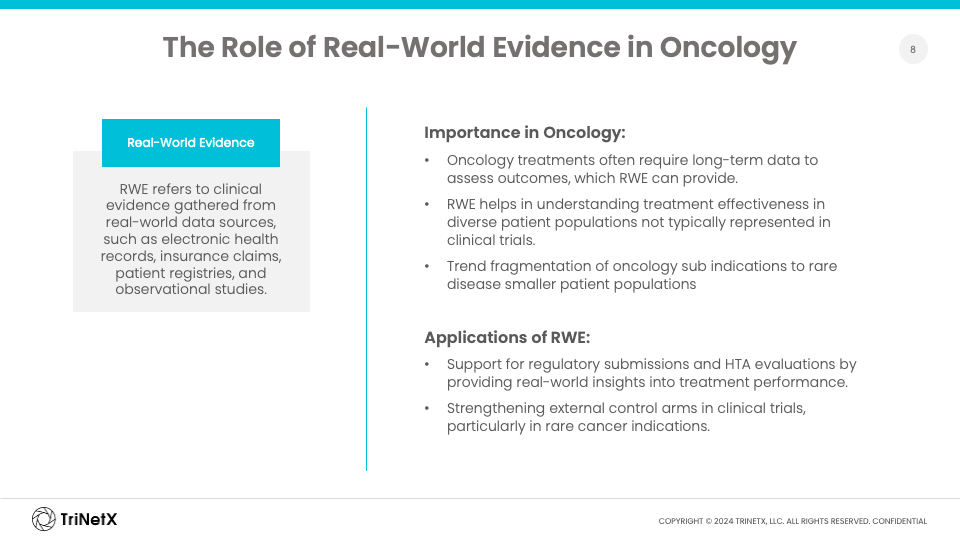
In recent years, there has been a significant shift in regulatory bodies’ acceptance of real-world data (RWD) and RWE. The EMA and FDA, among others, have issued guidelines emphasizing the importance of understanding how treatments perform outside clinical trials. This represents a growing trend towards patient-centered evidence, including patient-reported outcomes and quality of life measures, which now play a critical role in assessments.
Regulators are not just asking whether a treatment works in a clinical setting; they are increasingly interested in how these therapies impact patients’ daily lives and the broader healthcare ecosystem. For oncology treatments, in particular, where long-term data is essential, RWE helps assess outcomes and value across diverse and often underrepresented patient groups.
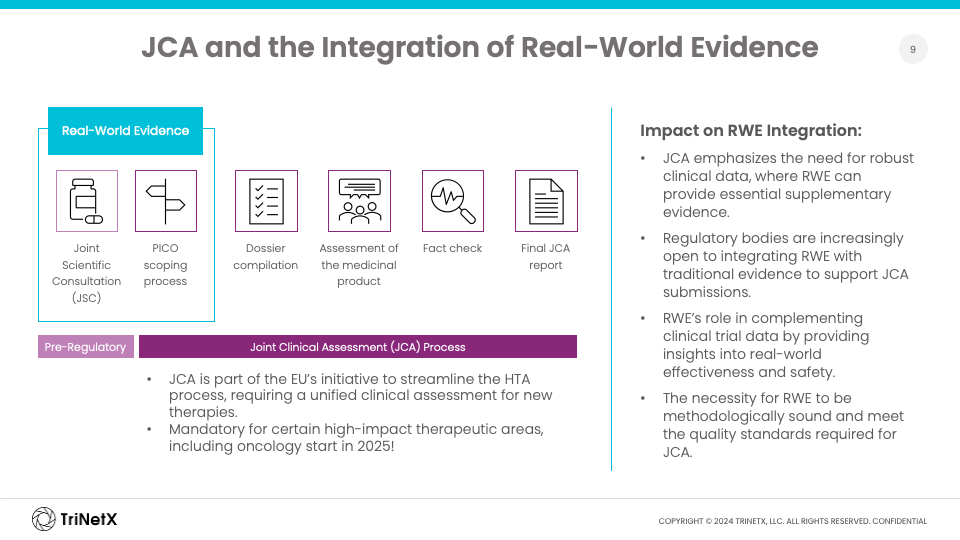
Joint Clinical Assessments and Harmonization Across Europe
One of the key developments in Europe is the introduction of mandatory joint clinical assessments (JCAs) under the new European regulation on HTA, set to begin in 2025. This regulation seeks to harmonize the clinical assessment process across European Union member states, starting with oncology, one of the high-impact therapeutic areas.
The JCA framework places a strong emphasis on robust clinical data, but this is where RWE can complement traditional data by providing essential supplementary evidence. RWD can offer insights into real-world effectiveness, safety, and patient outcomes, filling gaps that clinical trials may not adequately cover. The increasing openness of both regulatory and HTA bodies to the use of RWE marks a significant opportunity to meet evolving submission requirements.
The Importance of Data Quality and Early Engagement
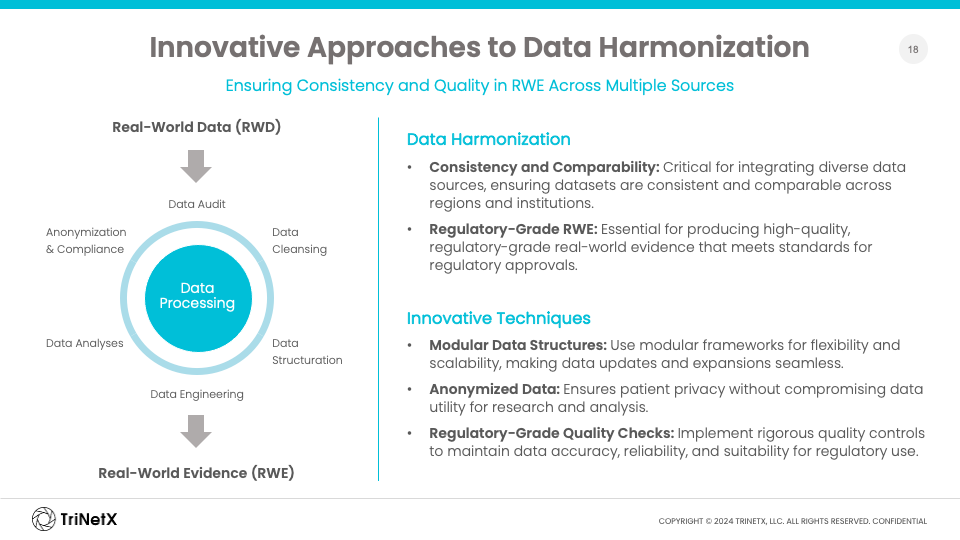
For RWE to be impactful in regulatory and HTA submissions, it must meet stringent data quality and methodological standards. This includes ensuring that data is fit for purpose and adheres to regulatory-grade requirements such as accuracy, completeness, and anonymization. Early engagement with regulatory bodies is also crucial to align expectations and ensure that RWE is effectively used to complement traditional data gaps.
Challenges such as data fragmentation, especially in Europe’s decentralized healthcare systems, pose significant hurdles. Navigating data privacy regulations like the General Data Protection Regulation (GDPR), ensuring compliance across various national laws, and securing representative patient samples are also critical concerns.
Additionally, data quality and completeness can vary significantly across regions, making standardization a priority. Regulatory-grade RWE must be methodologically sound to avoid issues such as bias, missing data, and inconsistency. The ability to access and integrate data quickly in response to regulatory questions is another key consideration for successful submissions.
“High quality always beats the number of data that you have, ensuring a fit with what regulators and HTA bodies want to see.” – Markus Rückert, PhD, TriNetX Oncology
TriNetX’s Strategic Use of RWE in Oncology
Oncology is uniquely suited for leveraging RWE due to the high fragmentation of treatments, with multiple sub-indications and rare disease niches. RWD allows researchers to understand how treatments perform in smaller, specific patient groups, such as older or frail patients, who are often underrepresented in clinical trials.
Case studies such as the HTA support for selinexor in Germany and the use of an external control arm for relapsed/refractory multiple myeloma patients demonstrate the power of RWE in oncology. The TriNetX Oncology team used advanced statistical techniques, such as inverse probability of treatment weighting and cox regression, to create reliable external control arms and generate meaningful, regulatory-grade data for decision-making.
“The analytical methods we used helped to produce reliable and clinically meaningful results, which contributed to better decision-making for treatment strategies.” – Zuzana Dostalova, TriNetX Oncology
Overcoming Data Collection Challenges in Oncology
One of the biggest challenges in collecting oncology data is the variability in treatment practices across different European countries, leading to inconsistent data. To address this, innovative approaches to data harmonization are essential. These include the use of modular data structures and rigorous quality checks to maintain accuracy and reliability across regions.
Securing diverse RWD through local collaborations and categorizing treating institutions by segments is key to ensuring representative samples. Achieving reliable data requires targeting over 10 percent of treated prevalence, reaching a 95 percent confidence level. This level of data consistency is necessary to overcome the fragmented nature of healthcare systems in Europe.
The Future of RWE in Oncology
The integration of RWE into oncology drug development and regulatory submissions is becoming increasingly important, particularly considering new European regulations like the JCA. As regulatory bodies continue to embrace RWD, the opportunity to supplement traditional clinical trials with RWE presents a path to more comprehensive and patient-centered decision-making.
To successfully leverage RWE, organizations must prioritize:
- Data quality,
- Early engagement with regulators, and
- Strategic use of data harmonization techniques.
By adopting these approaches, the full potential of RWE can be realized, helping to meet the demands of an evolving regulatory landscape in oncology, increase access to life-savings therapies, and ultimately improve patient outcomes.
Additional Insights:
- Access the full TriNetX webinar, Navigating the Evolving HTA & Regulatory Landscape in Oncology, on-demand.
- Read our blog, The Future of Oncology Research in Europe: Integrating Real-World Evidence into Joint Clinical Assessments.
- Explore TriNetX’s non-US oncology RWD services.
About Markus Rückert, PhD
Markus is a seasoned biopharmaceutical expert specializing in oncology and rare diseases. Proficient in clinical development and Medical Affairs, as well as experimental and RWE generation, Markus has successfully navigated various stages of drug development — from discovery to market launch — with a keen focus on advancing oncological and rare disease therapies for tangible patient benefits. Having achieved significant milestones in oncology and rare disease therapeutics, Markus now channels his diverse expertise towards broader pursuits in epidemiology, health service research, and the real-world impact of oncological treatments.
About Zuzana Dostalova
As a Data Scientist at TriNetX Oncology, Zuzana focuses on innovation and data quality to transform raw data into meaningful insights that support cancer research and patient care. By leveraging data science, she addresses challenges in oncology and ensures high standards of data accuracy and reliability. Zuzana collaborates closely with researchers, clinicians and other data analysts to produce scientifically robust and clinically applicable findings.