Clinical trials are the cornerstone of medical advancements, playing a pivotal role in the development of new treatments, interventions and understanding of diseases. Optimizing their success is crucial and real-world data (RWD) is increasingly recognized as a way to unlock key insights to improve protocol feasibility, site selection, and patient identification — helping to maximize efficiencies, reduce costs, and accelerate time to market.
Consider these facts:
- Recruitment and timeline misses — as many as 86 percent of clinical trials don’t meet their recruitment targets and up to 80 percent fail to finish on time.
- High cost of recruitment — on average, it costs $6,533 to recruit one patient to a clinical trial; if a patient is lost, it costs $19,533 to find a replacement.
- Unnecessary amendment delays and cost — amendments to protocols inflate costs, amounting to $600,000 to $8 million for every day of delay.
- Inefficient site identification — up to 50 percent of sites enroll 1 or no patients in their study.
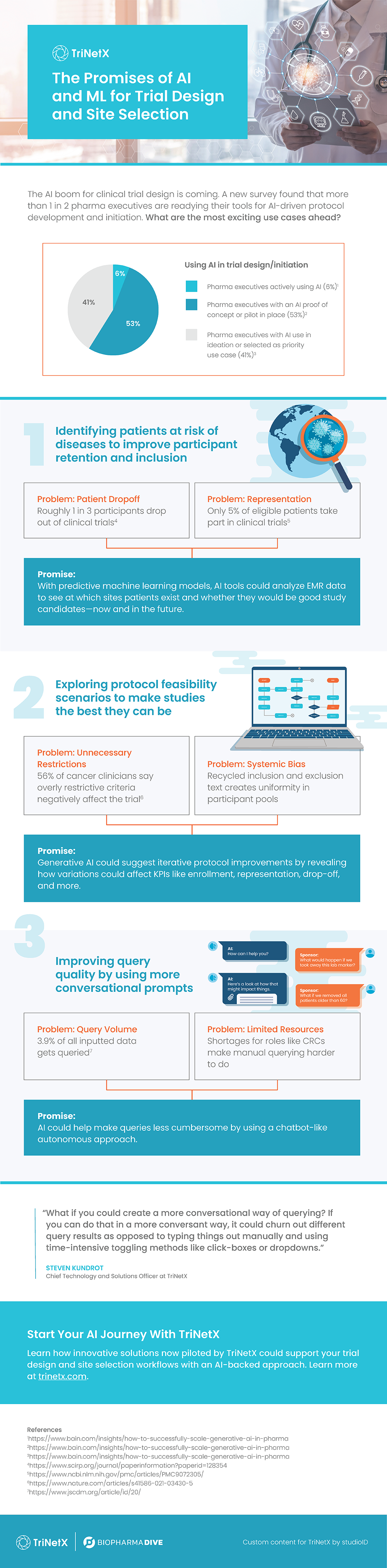
TriNetX has published a playbook, Beyond Tradition: Rethinking Clinical Trials with Real-World Data, detailing key strategies to help overcome these challenges and set clinical research up for success from the start.
Here are six reasons to dive into the playbook:
- Uncover how the right data foundation accelerates clinical trials by aligning study endpoints and engaging the right sites and participants.
- Discover how RWD tackles enrollment challenges, boosts diversity, and aligns with the U.S. Food and Drug Administration’s and global guidelines.
- Understand the importance of data integrity in accessing a diverse patient pool, providing a broader range of eligible participants across a variety of treatment settings.
- Learn how RWD ensures site preparedness, preventing delays by confirming sites have the necessary patient pools to target for recruitment.
- Explore how artificial intelligence (AI) and machine learning (ML), powered by RWD, revolutionize trial design and site selection.
- See the impact of RWD on the globalization of clinical trials, supporting their international expansion.
At TriNetX, we’re dedicated to empowering researchers around the globe to find answers to their clinical questions and drive scientific discoveries to improve patients’ lives. The pharmaceutical companies and life sciences organizations we partner with have discovered that incorporating RWD into their protocol feasibility, site selection, and patient identification processes results in better, more efficient trial design.
“By using TriNetX for feasibility, we have reduced the number of protocol amendments by 20 percent, now in line with industry average.”
“TriNetX is embedded into our company’s Center of Excellence’s protocol pressure testing. At least half the study teams have implemented at least one change over the last two years, observing reduction in avoidable protocol amendments due to optimized feasibility criteria, along with reduced cycle time.”
“We are starting to realize the potential of a RWD data source that connects back to patients and sites to impact the last mile of recruitment, as we have leveraged the TriNetX Network to engage with sites and TriNetX services to help identify over 80 patients for our digital waiting room.”
Ready to take your clinical trials to the next level? Get started by downloading our playbook – Beyond Tradition: Rethinking Clinical Trials with Real-World Data.

About Steve Kundrot
As Chief Technology and Solutions Officer at TriNetX, Steve is responsible for our trial design and optimization (TDO) business unit as well as technology, engineering, data management, platform operations, and cybersecurity across the company. Steve has a BS in Engineering from Cornell University and an MBA from Babson College.


