The landscape of clinical trials is evolving, and with it, the approach to site selection. A more patient-centric model is emerging, which flips the traditional process on its head.
Instead of beginning with known sites and then finding patients, this new approach starts by identifying the right patients through real-world data (RWD) and then determining the sites where they are already receiving treatment. This allows researchers to cast a wider net and identify potential trial participants from a broader geographic area and more diverse patient populations.
By focusing on where the right patients are, rather than trying to bring patients to where the known sites are, researchers can uncover untapped site potential, which could lead to more efficient, effective, and inclusive trials. That’s where TriNetX comes in.
TriNetX is empowering researchers with RWD that is seamlessly connected to their site selection process. This allows them to use patient counts as a predicting factor — down to the site level — to select their trial sites rather than rely solely on the traditional site identification approach, which is largely centered around convenience, infrastructure, and past performance.
Site-Level Patient Counts for More Precise Site Identification: The TriNetX Advantage
Currently, TriNetX has over 7,000 sites in the TriNetX LIVE™ platform, its healthcare RWD ecosystem. The sites are sourced from deidentified electronic health record (EHR) location data provided by over 180 healthcare organizations (HCOs) that are members of the TriNetX global network. A unique data processing funnel within the platform links EHR data to a patient encounter, which, in turn, is linked to a location address where that encounter occurred, and patient care is being delivered.
Three key benefits of this process include:
1. Improved Patient Recruitment
One of the most significant challenges in clinical trials is patient recruitment. Traditional methods often result in slow recruitment rates, leading to delays in trial timelines. By starting with TriNetX’s patient-centric approach, researchers can directly target sites where eligible patients are already receiving treatment. This not only speeds up the recruitment process but also ensures that the patient pool is diverse and representative of the real-world population.
2. Enhanced Site Selection
TriNetX’s patient-centric model enables researchers to discover sites that may not have been on their radar initially. These sites could be community hospitals, specialized clinics, primary care facilities, or other healthcare facilities that have access to the right patient population. This approach democratizes the site selection process, moving beyond the usual suspects to include a broader range of sites that can contribute valuable data to the trial.
3. Greater Inclusivity and Diversity
Traditional site selection processes can inadvertently exclude certain patient populations, particularly those in rural or underserved areas. The TriNetX approach, however, ensures that these populations are not overlooked. By identifying patients first, researchers can bring trials to them, rather than the other way around. Moreover, enhancing the inclusivity and diversity of trials can help with the submission of Diversity Action Plans, for which the U.S. Food and Drug Administration (FDA) issued draft guidance in June 2024.
TriNetX’s Site Identification Process in Action
The following example illustrates how a sponsor could utilize Site Dashboard, an automated tool within TriNetX LIVE™, to help identify sites for a phase III clinical trial.
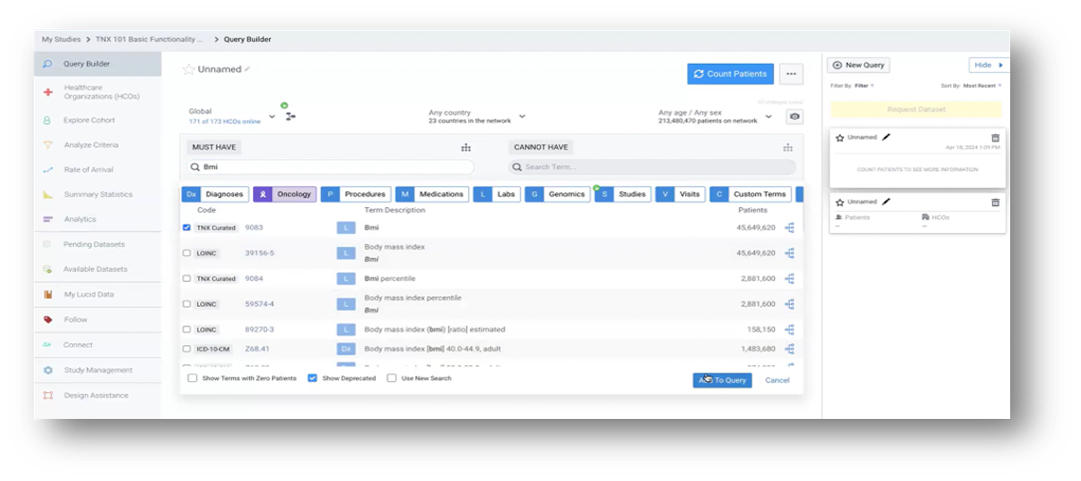
Source: TriNetX LIVE™ Site Dashboard
Step 1:
The sponsor translates the trial protocol into a set of inclusion/exclusion criteria, inputting a set of details (e.g., patient demographics, diagnosis codes, treatment history, other relevant factors) into the TriNetX LIVE™ platform. This allows for precise cohort building based upon the specific needs of the trial.
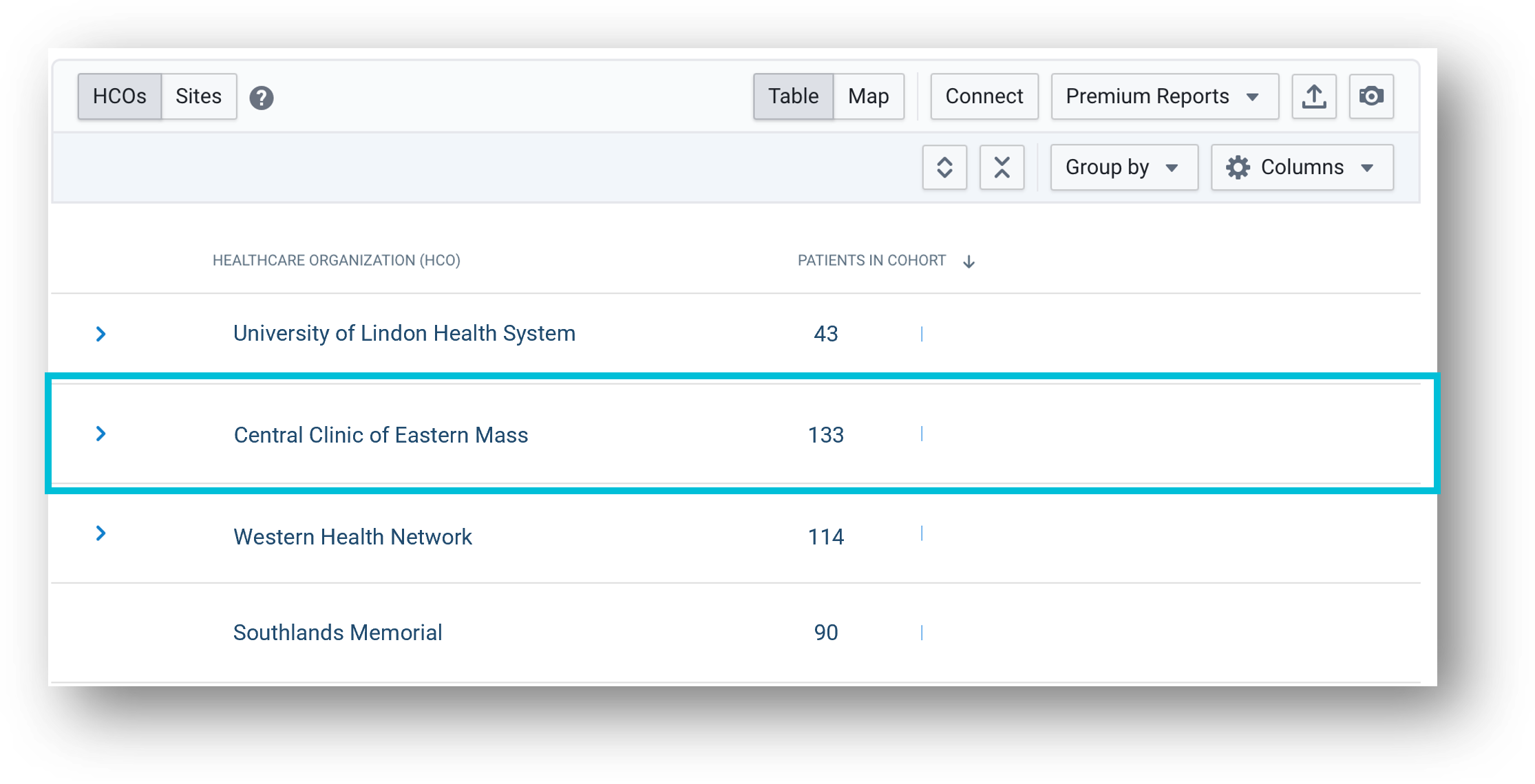
Source: TriNetX LIVE™ Site Dashboard. The names of organizations mentioned are fictitious and any resemblance to real entities is purely coincidental.
Step 2:
With the cohort built, the sponsor can see both the HCOs and the specific sites within each HCO’s network where patients receive care. In the figure below, the highlighted HCO has the most potentially eligible patients. Knowing more about the sites where these patients receive care will help the sponsor send highly targeted opportunities.
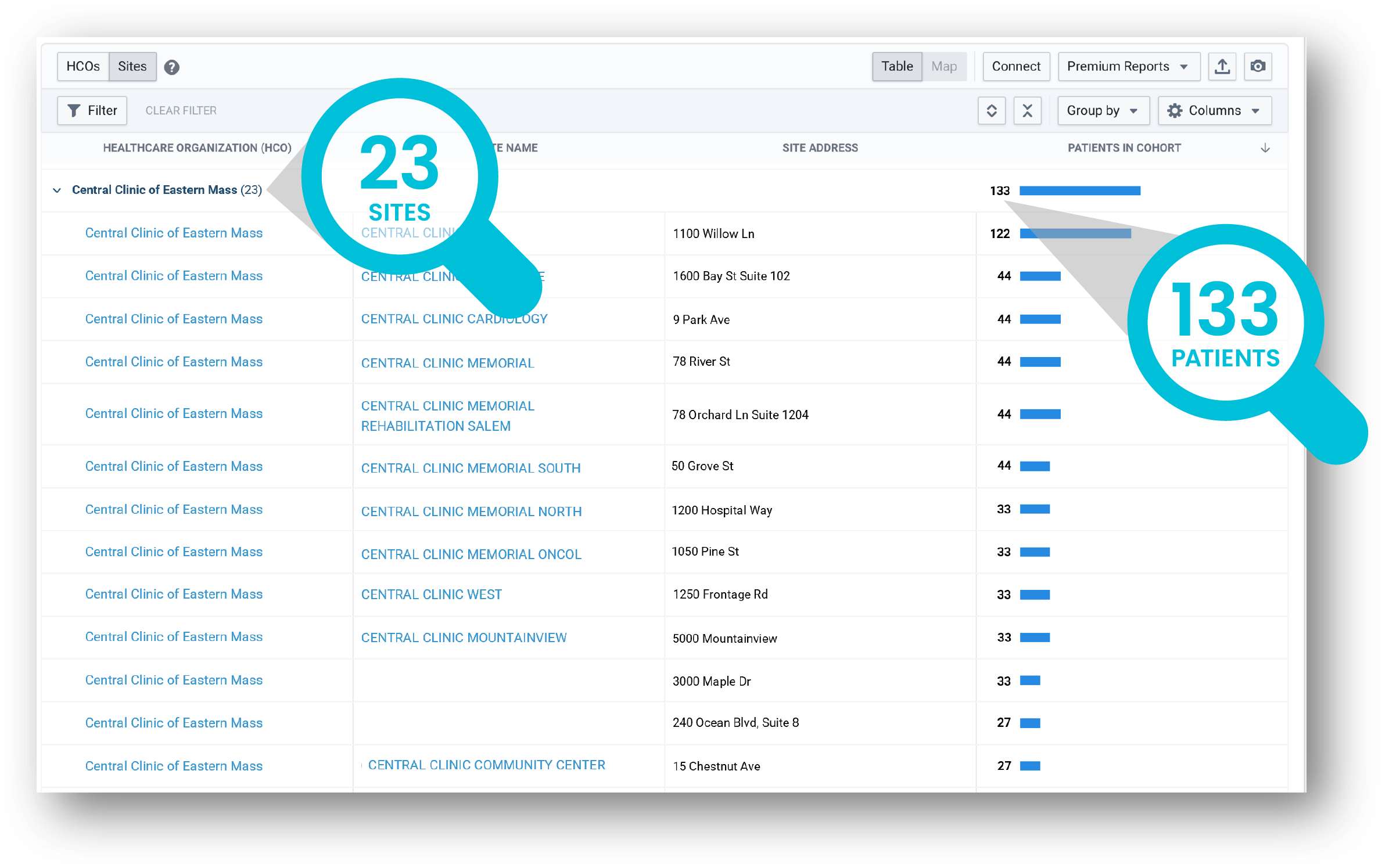
Source: TriNetX LIVE™ Site Dashboard. The names of organizations mentioned are fictitious and any resemblance to real entities is purely coincidental.
Step 3:
Going one layer deeper, the Site Dashboard identified 23 sites within the HCO treating 133 patients that meet the trial criteria.
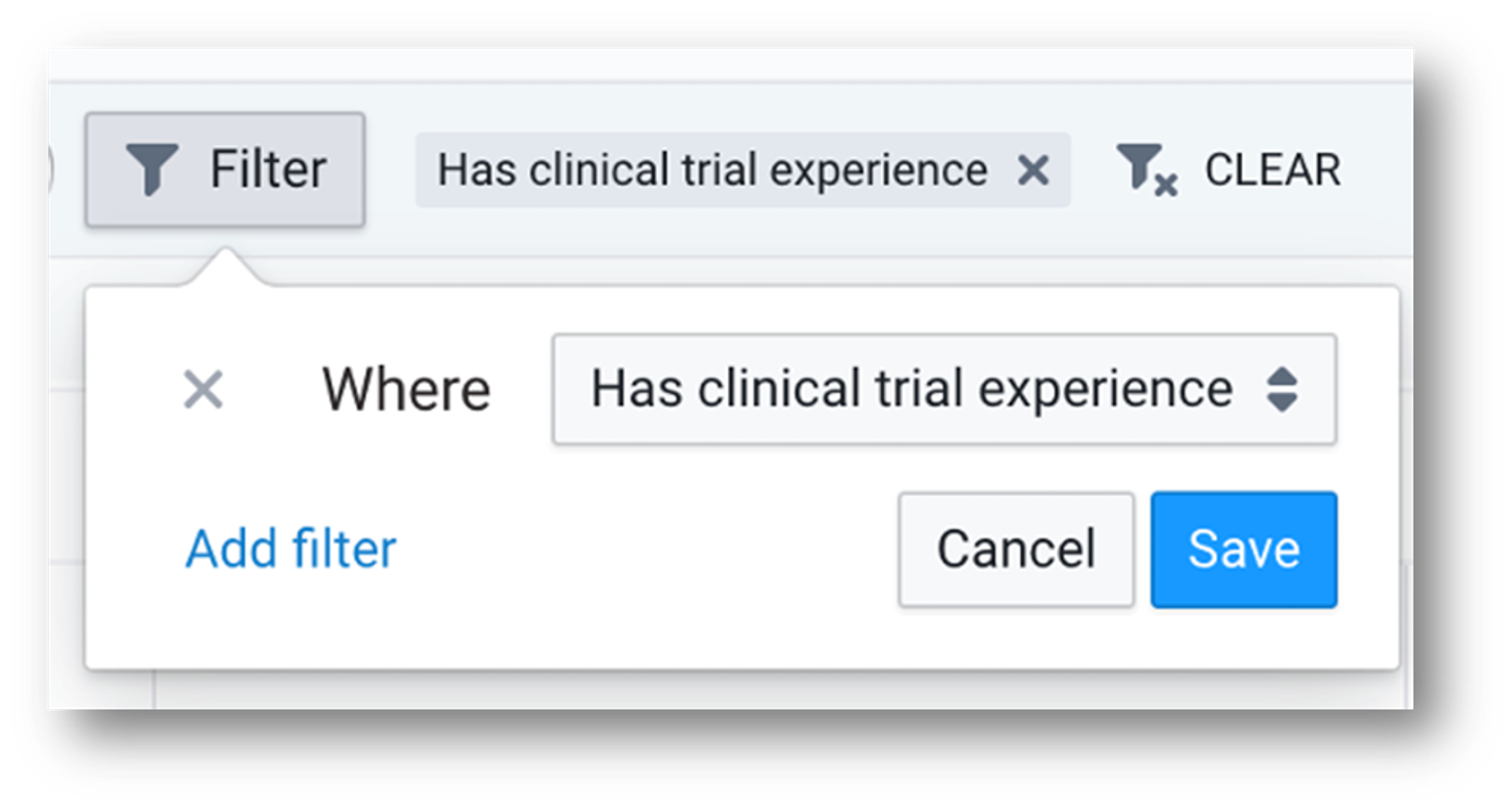
Source: TriNetX LIVE™ Site Dashboard
Step 4:
Using filters, sponsors can quickly pinpoint the sites within the HCO’s network actively participating in trials. This enables more targeted site identification by showing if there are patients that meet the protocol criteria at an active clinical trial site.
Rethinking Clinical Trial Site Selection for Better Clinical Research
At TriNetX, we see turning clinical trial site selection on its head not just as an opportunity but as a necessity. By leveraging RWD, we are helping the healthcare industry to create a more inclusive, efficient, and patient-centric approach to clinical research. This bold new model promises to not only improve trial outcomes but also drive forward medical innovation in a way that benefits all populations around the globe.
Want more? Explore TriNetX’s clinical trial design and optimization solutions.

About Justin North
Over the last decade, Justin has led dozens of software solutions from idea to commercial release with companies including Phillips Healthcare, goBalto, and Oracle. He brings a deep background in data science and clinical research to his role as Director of Product Management at TriNetX, where he has overseen the development of products for trial design and feasibility.

