“Diversity in clinical trials” is undoubtedly one of the hottest topics in the healthcare industry today and with good reason. Underrepresentation in clinical trials can have significant and wide-ranging consequences — impacting the quality and applicability of medical research, the safety and efficacy of treatments, and the overall health outcomes of underrepresented populations.
Yet, while clinical trial diversity is essential for creating effective, safe and appropriate healthcare solutions for all, people from racial and ethnic minority and other diverse groups continue to be underrepresented in clinical research.
This pervasive issue was tackled head on in a recent TriNetX webinar hosted by Jeffrey Brown, PhD, Chief Scientific Officer at TriNetX. Dr. Brown was joined by two industry experts — Sara Calvert, PharmD, Director of Projects at the Clinical Trials Transformation Initiative (CTTI) and Beth Brooks, MPH, Head of Patient Insights and Behavioral Sciences at Sanofi — for an insightful discussion on the use of real-world data (RWD) to enhance clinical trial design, promote health equity and foster robust evidence generation.
Continue reading for conversation highlights and access the TriNetX webinar on-demand for the full discussion.
Moving the Needle Toward More Representative Clinical Trials
Founded in 2007, CTTI is a public-private partnership that actively engages with its member organizations to enhance the quality and efficiency of clinical trials. Through diverse projects and collaborations, CTTI aims to address and solve critical issues within clinical trials by promoting innovative practices and overcoming existing barriers to participation. These efforts are supported by a combination of research, literature reviews and expert meetings, which facilitate the sharing of implementation examples and best practices.
CTTI’s Dr. Calvert kicked the webinar off by providing an overview of the landscape of diversity in clinical trials today, emphasizing:
- Efforts to drive adoption of practices aimed at increasing the quality and efficiency of clinical trials, including the decades-long commitment and work of the U.S. Food and Drug Administration (FDA) to enhance the diversity of clinical trial populations in the U.S.
- The passage of the Food and Drug Omnibus Reform Act (FDORA) in 2022 requiring trial sponsors to submit diversity action plans for all Phase 3 trials and other pivotal trials.
- The FDORA public workshop, held in 2023, focusing on how to reach clinical study enrollment goals through innovative approaches including technology-driven decentralized clinical trials (DCTs) and efforts to foster greater community engagement to support inclusion.
While progress is being made, according to Dr. Calvert, deep organizational commitment is needed to develop a clinical trial infrastructure that supports the needs of historically underrepresented populations — an infrastructure that ensures the full clinical trials ecosystem is working together, utilizing data-driven strategies and constantly striving for improvement.
Dr. Calvert also shared details on several projects CTTI has undertaken to improve diversity efforts. One of these is the development and implementation of a national action plan in collaboration with hundreds of leaders in health equity and representation.
“We’ve all done work in this space,” she said. “What can we do to improve diversity in clinical trials in a way that can’t be done with everybody working alone?”
The national action plan includes eight key areas of focus to achieve better representation in clinical trials:
- Public Awareness and Communication
- Community Engagement
- Site Enablement
- Workforce Diversity Accountability
- Comprehensive Data
- Funding
- Resources and Support
- Trial Participation Access
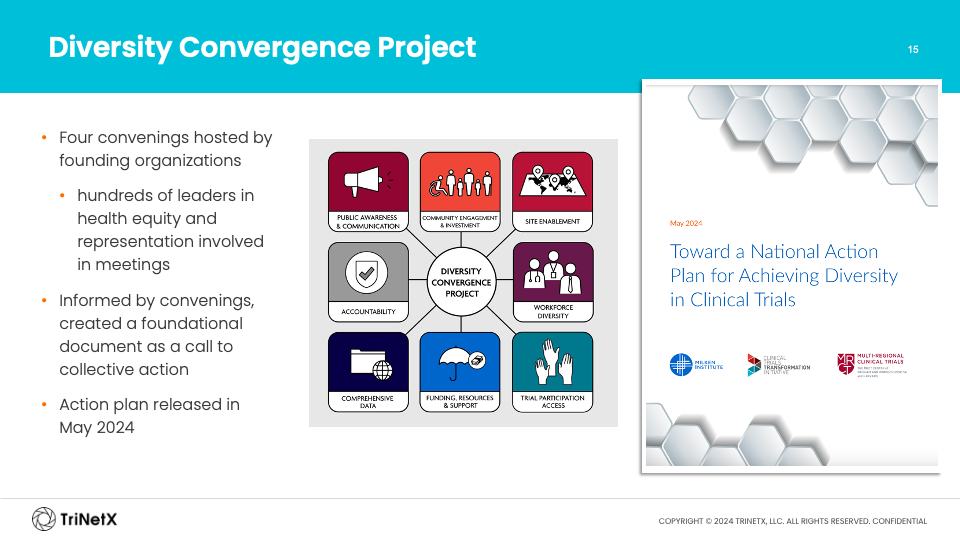
With the plan solidified, the team is beginning the necessary work in each of the eight domains to further drive clinical trial diversity forward.
Unlocking the Power of RWD: Sanofi’s Approach to Improving Clinical Trial Representation
Sanofi is at the forefront of integrating diversity within its clinical trials, emphasizing a robust internal commitment to diversity, equity and inclusion. This commitment is reflected organization-wide and rigorously applied to their design and execution of clinical trials.
By leveraging RWD from TriNetX, Sanofi develops a nuanced understanding of disease prevalence across diverse populations, which informs the setting of realistic and inclusive participant goals. According to Sanofi’s Beth Brooks, a key strategy involves annual reporting on the inclusiveness of clinical trials, assessing how representative these trials are compared to the broader population.
Sanofi sets diversity goals for each trial, with a strategic plan to expand this practice globally. RWD is crucial in identifying restrictive criteria that might limit the participation of diverse groups, allowing for adjustments in real-time to enhance inclusiveness, Beth explained.
Beth’s presentation transitioned to what information should be put into a clinical trial diversity plan and the types of goals that should be set, noting that this is a common struggle among trial sponsors.
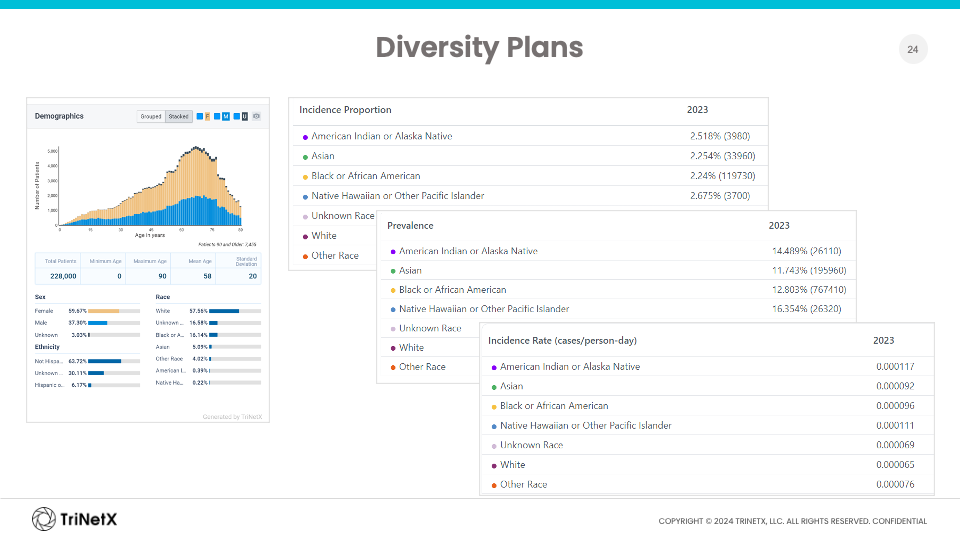
She shared that Sanofi utilizes RWD extracts from TriNetX along with data and analytics from other sources to set recruitment goals, see how factors such as incidence proportion differ across various racial and ethnic groups, and assess how these factors might affect their clinical trial recruitment strategy — all with the aim of keeping barriers down and ensuring they are being as inclusive as possible.
“This is still a work in progress for us,” Beth said. “We’ve done perhaps six or seven of these and there are still more questions than answers when it comes to diversity plans. We haven’t gotten a lot of feedback from the FDA yet. We’re eagerly anticipating their final guidance and hopefully we’ll be able to see more of what we’re expected to do.”
Clinical Trial Diversity: A Complex Problem, No Simple Solution
The experts’ presentations underscore the fact that while advancements concerning diversity in clinical trials have been made, challenges remain.
Collaborative efforts and strategic commitments, both at the organizational and industry levels, were recognized as essential for driving continuous improvement and ensuring trials are inclusive and representative.
Additionally, RWD was acknowledged as a critical tool for setting realistic diversity goals and adjusting clinical trial criteria to better reflect diverse populations. “TriNetX has been doing a lot of work in this space,” explained Dr. Brown. “Obviously, we’re trying to get better and better. Our job is to get data and technology in the hands of those who can use it best — in this case, use it best for clinical trial planning and in the end, getting trials that reflect the appropriate population, the right population.”
Advancing diversity in clinical trials isn’t just a matter of equity; it’s a scientific, ethical and regulatory necessity. As the healthcare landscape continues to evolve, embracing data-driven approaches will be crucial in building a more inclusive and effective research paradigm.
Additional Insights:
- Access the full TriNetX Diversity in Clinical Trials webinar, including the Q&A session, on-demand.
- Read the TriNetX blog, Real-World Data to Improve Clinical Trial Design.
- Explore TriNetX’s clinical trial diversity solutions.